Chapter 55 Epilepsy and Neurodevelopmental Disorders
Introduction
The association of epilepsy with neurodevelopmental disorders is now well established. Children with developmental epilepsies are at increased risk for cognitive [Hermann and Seidenberg, 2007], neurobehavioral [Hermann et al., 2008], and psychiatric disorders [Plioplys et al., 2007]. Specific factors associated with a higher risk of neuropsychological deficits in children with epilepsy include multiple seizures, use of antiepileptic drugs, etiology, and interictal epileptiform discharges (IEDs) on the initial electroencephalogram (EEG) [Fastenau et al., 2009]. In children with epilepsy, younger age at seizure onset, cognitive impairment, temporal or frontal lobe onset of seizures, and intractable epilepsy are associated with an increased likelihood of coexisting social, communication, and behavioral disorders [Hamiwka and Wirrell, 2009]. The multiple complex and confounding risk factors that account for the coexistence of epilepsy and neurodevelopmental disorders include etiology, associated disabilities, seizure type, frequency, duration, control, age of onset, and IEDs, as well as psychosocial factors and medication effects [Austin and Caplan, 2007].
The relationship between epilepsy and neurodevelopmental disorders also reflects common pathologies and mechanisms [Tuchman et al., 2009], and there is accumulating evidence to suggest that cognitive and behavioral impairments may precede the onset of seizures [Austin et al., 2001; Oostrom et al., 2003; Bhise et al., 2009; Fastenau et al., 2009]. On the other hand, there is evidence that recurrent seizures or IEDs can cause acute and long-lasting impairment of brain function and development [Holmes and Lenck-Santini, 2006; Galanopoulou and Moshe, 2009], and significant efforts are being made to determine how risk factors contribute to developmental outcomes in children with epilepsy [Austin and Fastenau, 2009]. The notion that epileptic activity, seizures, or IEDs can lead to cognitive and behavioral impairment above and beyond what might be expected from the underlying pathology is exemplified by the concept of epileptic encephalopathy [Berg et al., 2009]. This idea has important implications for treatment, especially in children with an epileptic encephalopathy, in whom early and successful treatment of seizures and interictal epileptiform activity may be crucial to positive neurodevelopmental outcomes [Freitag and Tuxhorn, 2005; Jonas et al., 2005; Lux et al., 2005; Arts and Geerts, 2009; Bombardieri et al., 2009].
Epilepsy is more frequent in children with intellectual disability [Goulden et al., 1991]; the behavioral, cognitive, and social aspects of epilepsy are multiple and diverse, and are discussed in Chapter 62. This present chapter focuses on the concept of epileptic encephalopathy and the association of epilepsy and IEDs with specific language impairments and autistic spectrum disorders (ASDs). In addition, the common mechanisms of disease and management of children with developmental epilepsies and neurodevelopmental disorders are addressed.
Epileptic Encephalopathies
The epileptic encephalopathies can occur at any age, but are more common and severe in the first decade of life as the brain is developing. Approximately 40 percent of epilepsies occurring during the first 3 years of life are associated with an epileptic encephalopathy [Guerrini, 2006]. Epileptic encephalopathy of childhood includes: early myoclonic encephalopathy (Ohtahara’s or Aicardi’s syndrome), severe myoclonic epilepsy of infancy (Dravet’s syndrome), myoclonic-astatic epilepsy of early childhood (Doose’s syndrome), infantile spasms (West’s syndrome), Lennox–Gastaut syndrome, and Landau–Kleffner syndrome-continuous spike waves during slow-wave sleep [Engel, 2001].
The myoclonic epilepsies and infantile spasms are discussed in Chapter 56 and Lennox–Gastaut syndrome in Chapter 53. In this chapter, Dravet’s syndrome, infantile spasms, Lennox–Gastaut syndrome, Landau–Kleffner syndrome, and continuous spike waves during slow-wave sleep will be discussed from a developmental perspective. Specifically, the impact of seizures and of IEDs on the developmental trajectories of children with these electroclinical syndromes will be emphasized.
Dravet’s Syndrome
Dravet’s syndrome is a genetically determined infantile epileptic encephalopathy, mainly caused by de novo mutations in the SCN1A gene [Scheffer et al., 2009]. Early development is normal, with seizures usually beginning in the first 10 months of life; these become frequent by 2–4 years of age, with focal and generalized myoclonus, atypical absences, and partial complex seizures, as well as seizures characterized by fluctuating alteration of consciousness with reduced postural tone and myoclonic jerks [Millichap et al., 2009]. Despite frequent seizures, the EEG is usually normal in the first 2 years of life and then usually progresses into spike and wave and multifocal discharges, although some children may have persistently normal interictal EEG studies [Korff et al., 2007]. Progressive decline or plateau in development occurs by 1–4 years of age, with intellectual disability and an autism phenotype commonly present, especially in those with more than five seizures per month [Scheffer et al., 2009].
Genes associated with Dravet’s syndrome appear to have relevance to the neurodevelopmental disorders, such as ASDs. Specifically, a susceptibility locus for ASDs has been found on chromosome 2 in the vicinity of the epilepsy-involved genes SCN1A and SCN2A [Weiss et al., 2003], while PCDH10 on chromosome 4 has been linked to ASDs in families with shared ancestry [Morrow et al., 2008]. Recently, a sporadic infantile epileptic encephalopathy that resembles Dravet’s syndrome has been tied to mutations in PCDH19 [Depienne et al., 2009]. These results hint strongly at possible shared molecular underpinnings between ASD and this epileptic encephalopathy.
Infantile Spasms
Infantile spasms constitute an age-specific seizure disorder, with a peak age of presentation between 4 and 8 months of age, a critical time of brain development [Zupanc, 2009]. There is a strong correlation between infantile spasms and intellectual disability [Trevathan et al., 1999], and the majority of children with infantile spasms develop intellectual disability and specific cognitive and behavioral deficits [Riikonen, 2001]. In addition, the association of infantile spasms and ASDs is well recognized [Riikonen and Amnell, 1981]; in children with infantile spasms the prevalence of ASDs is as high as 35 percent, depending on the severity of intellectual disability [Saemundsen et al., 2007], with a heightened risk of ASDs in the presence of identifiable structural lesions of the brain [Saemundsen et al., 2008]. Further, in children with infantile spasms, EEG epileptiform activity – particularly bilateral frontal EEG discharges and persistence of hypsarrhythmia – contributes to the development of the autism phenotype [Kayaalp et al., 2007].
One of the intriguing features of infantile spasms is that the severity and, to a certain extent, the frequency of seizures do not seem to correlate with the degree of cognitive impairment or the occurrence of regression [Riikonen, 1984; Bednarek et al., 1998]. It may be that the interictal EEG pattern of hypsarrhythmia associated with infantile spasms affects cortical and subcortical neuronal networks, resulting in abnormal synaptogenesis and poor developmental outcomes [Zupanc, 2009].
Lennox–Gastaut Syndrome
Lennox–Gastaut syndrome is an age-specific epileptic syndrome that peaks between 3 and 5 years; it is characterized by multiple seizure types that include tonic and atonic seizures, atypical absences, and myoclonic and generalized or focal seizures, in association with a characteristic EEG pattern of slow spike-and-wave complexes, often associated with multifocal epileptiform activity and runs of fast activity [Crumrine, 2002]. Approximately 20 percent of children with this epileptic syndrome have a history of infantile spasms [Markand, 2003]. Cognitive and behavioral abnormalities precede the clinical seizures in approximately 20–60 percent of children with Lennox–Gastaut syndrome [Blume, 2001].
It has been suggested that the epileptic processes associated with this syndrome lead to patterns of abnormal activity and connectivity that compete with normal brain development, thus resulting in subsequent impairment or regression of cognition [Blume, 2004]. It is not known whether it is the underlying brain pathology, the burden of frequent seizures, the persistent epileptiform activity, or all of these factors that is responsible for the cognitive deficits in Lennox–Gastaut syndrome. The age specificity, typically frequent seizures, and unremitting epileptiform activity suggest that the epileptic activity occurring at a critical time in brain development contributes to a progressive disturbance in cerebral function.
Landau–Kleffner Syndrome-Continuous Spike Waves During Slow-Wave Sleep
Landau–Kleffner syndrome (LKS) is an acquired aphasia associated with an epileptiform EEG with spikes, sharp waves, or spike-and-wave discharges that are usually bilateral and occur predominantly over the temporal regions; in approximately 25 percent of children there are no clinical seizures [Landau and Kleffner, 1998]. Continuous spike-wave discharges during slow-wave sleep (CSWS), an epileptic encephalopathy associated with the EEG pattern of electrical status epilepticus during slow-wave sleep, with various seizure types, and with cognitive, motor, and behavioral disturbances, is, along with LKS, considered a sleep-related epileptic encephalopathy [Tassinari et al., 2009].
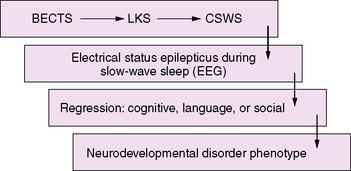
CSWS and LKS are epileptic encephalopathies with common clinical features, including seizures, regression, and epileptiform abnormalities that are activated by sleep [Nickels and Wirrell, 2008]. In CSWS there is a regression in global skills, while in LKS the primary clinical manifestation is a regression of language. The conceptual thinking is that there is a spectrum of disorders associated with activation of epileptiform activity during slow-wave sleep that includes LKS, CSWS, and “atypical” benign epilepsy with centrotemporal spikes (BECTS) [Fejerman, 2009; Scheltens-de Boer, 2009]. The implications are that the EEG abnormalities lead to regression in language, behavioral, and cognitive manifestations and that treatment requires reversal of the epileptiform pattern on the EEG. However, whether the frequency of the epileptiform discharges, as linked to the appearance or disappearance of electrical status epilepticus during slow-wave sleep, is responsible for the course of language, cognitive, and behavioral deterioration [Tassinari et al., 2000; Seri et al., 2009] remains an unanswered and controversial issue.
Seizures, Interictal Epileptiform Discharges, and Neurodevelopmental Disorders
IEDs, characterized by spikes or sharp waves that appear abruptly from the electrographic background, with or without an associated slow wave, are limited in duration and do not evolve in frequency and distribution over time, but can at times be responsible for disruption of cognitive and behavioral function [Fisch, 2003]. The concept that transitory changes in higher cortical functions can be secondary to EEG discharges not accompanied by seizures was proposed more than 60 years ago [Schwab, 1939]. The term transient cognitive impairment is used to describe individuals with epileptiform EEG discharges in association with a momentary disruption of adaptive cerebral function [Aarts et al., 1984; Binnie, 2003].
Studies by numerous investigators suggest that transient cognitive impairment is not a consequence of general impairment of attention, but is likely secondary to disruption of functions located in the region or regions of the brain where the epileptiform discharges arise [Kasteleijn-Nolst Trenite, 1995; Aldenkamp and Arends, 2004]. Specific functions, such as perception, reaction time, and scholastic performance, can be disrupted by brief epileptiform discharges in the absence of convulsive seizures [Shewmon and Erwin, 1988; Sengoku et al., 1990]. The spectrum of language and cognitive impairment secondary to an active epileptic focus, even in the absence of clinical seizures, is wide [Deonna and Roulet-Perez, 2005; Roulet-Perez and Deonna, 2006].
Specific Language Impairment
Specific language impairment is a developmental language disorder characterized by varying types and degree of dysfunction in expressive and receptive communication skills (see Chapter 45). Children with specific language impairments differ from children with acquired aphasia characteristic of the epileptic encephalopathies, such as LKS-CSWS, in that there is no regression of language skills. Nevertheless, the impairments of language in epilepsies such as BECTS raises interesting questions regarding the overlap and interactions of epilepsy, IEDs, and specific language impairments [Billard et al., 2009].
An increased association of seizures in children with specific language impairments has been found [Robinson, 1991; Echenne et al., 1992], with prevalence rates from 7–40 percent reflecting the cohort studied and the type and duration of the EEG recording [Parry-Fielder et al., 1997]. In one study that compared the rates of epilepsy in children with autistic spectrum versus developmental language disorders, the prevalence of epilepsy was 8 percent (14 of 168) in the nonautistic, language-impaired children. In addition, when the children were subtyped on the basis of language, the highest risk of epilepsy, regardless of whether the children had ASD or specific language impairment, was seen in those individuals with the most severe receptive language disorder [Tuchman et al., 1991; Klein et al., 2000]. A link between specific language impairment and IEDs during sleep has also been suggested [Parry-Fielder et al., 1997; Ballaban-Gil and Tuchman, 2000; Wheless et al., 2002]. However, the strength of this association has been questioned in a recent study in which 13 percent of children with specific language impairments had IEDs on a sleep EEG recording, which was not significantly different than the non-language-impaired control group [Parry-Fielder et al., 2009].
Autistic Spectrum Disorders
ASD is a broad classification that includes a heterogeneous group of individuals with behaviorally defined impairments in reciprocal social interaction, verbal and nonverbal communication, and restricted and repetitive behaviors (see Chapter 48). The prevalence of epilepsy in children with ASD is highly variable, depending on the study sample, with rates ranging from 5 to 46 percent [Spence and Schneider, 2009]. The major risk factor for epilepsy in children with ASD is moderate to severe intellectual disability [Amiet et al., 2008]. In children with ASD without severe intellectual disability, motor deficit, associated perinatal or medical disorder, or a positive family history of epilepsy, the prevalence of epilepsy is 6 percent, which is not significantly different than in nonautistic children with a specific language impairment [Tuchman et al., 1991]. The prevalence of ASD in individuals with epilepsy has not been investigated with the same intensity as that of epilepsy in ASD, although one study in a tertiary epilepsy clinic found that approximately 30 percent of children with epilepsy screened positive for ASD [Clarke et al., 2005].
The prevalence of IEDs in children with ASD and no clinical history of seizures may be as high as 60 percent [Chez et al., 2006], but varies depending on the cohort studied and the type of EEG performed, with most studies finding prevalence rates ranging from 6 to 31 percent [Kagan-Kushnir et al., 2005]. The higher prevalence of IEDs in children with specific language impairment and in those with ASD compared to the 1.5–4 percent rate of IEDs in the general population [Cavazzuti et al., 1980; Capdevila et al., 2008] has been a source of significant controversy. The significance of these findings remains unclear and is not unique, as IEDs have been reported in 6–30 percent of children with attention-deficit hyperactivity disorders [Hughes et al., 2000; Richer et al., 2002; Kaufmann et al., 2009], which is remarkably similar to the prevalence of IEDs in children with specific language impairment or ASDs without seizures. One suggestion is that the high prevalence of IEDs in all of these disorders is secondary to the neuropathological processes common to both epilepsy and neurodevelopmental disorders. However, the question of whether these IEDs may be a biomarker of prognostic or interventional significance in a subgroup of children with specific language impairment or with ASDs remains to be determined.
Regression
The association of developmental regression in ASD to epilepsy and IEDs became a topic of increased interest after Hagberg and colleagues [Hagberg et al., 1983] published a report of 35 girls with regression in higher brain functions, stereotypical hand movements, and ASD, with a significant proportion of the girls having epilepsy. Childhood disintegrative disorder is characterized by late-onset autistic and cognitive regression that can include motor regression and loss of bowel and bladder function, usually occurring after age 3 [Rapin, 1995]. Childhood disintegrative disorder is based on the description, in the early 1900s, of children with normal development until age 3 or 4 years, who regressed in multiple developmental areas. This entity was first described by Heller in 1908 [Mouridsen, 2003], and differentiation from autism, especially from autistic regression, is still in progress [Kurita et al., 2004]. Disintegrative disorder is very rare, with a prevalence of 2 in 100,000 [Fombonne, 2009].
The prevalence of epilepsy in Rett’s syndrome and in childhood disintegrative disorder is greater than 70 percent, both disorders have EEGs with marked IEDs, and both featuring regression of cognitive, language, and social skills [Mouridsen et al., 1999; Steffenburg et al., 2001]. Some children with childhood disintegrative disorder overlap with those having epilepsy and CSWS [Roulet Perez et al., 1993]. To what extent the high rate of seizures in these groups is secondary to the severe cognitive impairment present in Rett’s syndrome and childhood disintegrative disorder, or what influence other specific variables, such as metabolic or molecular factors (i.e., the role of MECP2), have in the development of seizures remains unknown.
The developmental trajectory in approximately 30 percent of children with ASD is characterized by a regression of the few words acquired and a loss of nonverbal communication skills, usually occurring prior to 24 months of age [Goldberg et al., 2003; Lord et al., 2004]. This regression has been termed autistic regression. The relation of autistic regression to epilepsy or to an epileptiform EEG without seizures remains controversial, with some studies reporting higher rates of epilepsy in children with ASD and regression [Kobayashi and Murata, 1998; Hrdlicka et al., 2004], and others showing no relation between ASD, epilepsy, and regression [Tuchman and Rapin, 1997; Baird et al., 2008]. A recent study found that children with autistic regression had more disrupted sleep, as compared to those with autism without regression, and were more likely to have epilepsy [Giannotti et al., 2008]. In addition, Giannotti and colleagues found that epileptiform activity did not differ among those with and without regression, except that those with autistic regression were more likely than those without regression to have more “frequent epileptiform EEGs.” There is evidence to suggest that, in a subgroup of children with ASD and without convulsive seizures, an epileptiform EEG is significantly more likely to be associated with a history of regression in language [Tuchman and Rapin, 1997]. However, these data must be put into perspective, as they represent a very specific subgroup of children with autism, and because at the present time there are no data regarding the number of children in the general population without seizures and cognitive and behavioral impairments who have interictal epileptiform abnormalities on an overnight EEG study. Others have found no differences in regression in those with epileptiform EEGs and epilepsy and those without seizures and a normal EEG [Canitano et al., 2005]. Children with autistic regression and an epileptiform EEG (AREE) should be differentiated from those with LKS-CSWS, and these differences are highlighted in Table 55-1.
Table 55-1 Landau–Kleffner Syndrome-Continuous Spike Waves During Slow-Wave Sleep Versus Autistic Regression with Epileptiform EEG
| Landau–Kleffner Syndrome-Continuous Spike-Waves During Slow-Wave Sleep (LKS-CSWS) | Autistic Regression with Epileptiform EEG (AREE) | |
|---|---|---|
| Age of regression/(symptoms) | Usually after 3 years. Peak age 3–5 years. In CSWS may be as late as 12 years of age | Usually prior to age 2 years with a mean age of regression of 21 months |
| Seizures | Usually not frequent or intractable. In approximately 25 percent, seizures are not present | Seizures are not part of phenotype. In autistic spectrum disorders, when seizures occur, they are usually not frequent and responded well to antiepileptic medications |
| EEG | Spikes, sharp waves, or spike-and-wave discharges, usually bilateral and occurring predominantly over the temporal regions. They increase during sleep; EEG pattern of electrical status epilepticus during slow-wave sleep is common | Infrequent spikes, usually centrotemporal. Rarely associated with CSWS. No clear correlation with interictal epileptiform discharges and improvement or worsening of underlying language and social dysfunction |
| Treatment | See Box 55-1. Case reports, mostly with use of steroids, suggest improvement in language. Surgical outcomes with multiple subpial transections are variable. No controlled clinical trials | No evidence that present medical interventions (antiepileptic medications) or surgical interventions are indicated |
| Outcome/Comments | Improvement occurs in late childhood/early adolescence. Approximately one-third recover. Prognosis for seizure control is excellent but recovery of language is variable and not as good as for seizures | Improvement seems related to cognitive skills. No data to determine if interictal discharges combined with regression are marker for worse prognosis |
The mean age of onset of language regression in ASDs is 21 months, and over 90 percent of children with autism who undergo a regression do so before age 3 years [Tuchman and Rapin, 1997]. By contrast, in LKS, only 12–14 percent of children experience regression before age 3 years [Bishop, 1985]. The peak age of onset of symptoms in LKS is between 5 and 7 years [Bureau, 1995]. In only 5 percent of individuals does LKS begin after age 9 years and it appears to occur rarely, if ever, after age 12 years [Bureau, 1995]. In children with the CSWS, the first symptoms occur in up to 20 percent of children between 9 and 12 years of age [Bureau, 1995].
Children with ASD are more likely to regress earlier, usually prior to age 2, as contrasted to those with LKS who have a regression in language usually after age 3 years, and seizures are more likely to occur in children who regress in language after age 3 years [Klein et al., 2000; Shinnar et al., 2001; Wilson et al., 2003]. In addition, children with isolated language regression have a higher frequency of epileptiform discharges and seizures than children with both language and autistic (social and behavioral) regression [McVicar et al., 2005]. Furthermore, electrical status epilepticus during slow-wave sleep, the EEG pattern associated with LKS and CSWS, is almost exclusively found in those children with isolated language regression [McVicar et al., 2005], and CSWS with autistic regression is a rare occurrence [Tuchman, 2009]. The age of symptom onset seems to be an important indicator of outcome, at least in LKS. In one study, the prognosis for recovery was worse in children with LKS who lost their language at an early age [Bishop, 1985]. In a series of studies, age of language regression differentiates autistic regression from LKS.
In LKS, improvement occurs usually toward late childhood or early adolescence. Approximately one-third of affected children make a good recovery [Mantovani and Landau, 1980]. The prognosis for seizure control and normalization of the EEG is excellent, but prognosis for recovery of language and cognitive function is variable and in general not as good as it is for the seizures [Smith and Hoeppner, 2003]. In the group of children with regression and global cognitive deficits in the context of electrical status epilepticus during slow-wave sleep (ESES), a significant majority is left with some degree of neurological impairment [Rossi et al., 1999; Robinson et al., 2001]. In childhood disintegrative disorder, the prognosis is generally poor [Gillberg, 1991]. In general, children with autistic regression have significant long-term morbidity [Wilson et al., 2003]. What is still not known is whether children with autistic epileptiform regression have a poorer prognosis than those with regression without an epileptiform EEG.
An emerging concept of epileptic encephalopathy suggests a continuum of disorders in which the interictal epileptiform activity activated by sleep may account for regression in varying neurodevelopmental domains (Figure 55-1). This hypothetical model may provide a framework to understand the impact of seizures and interictal epileptiform activity on the developing brain, as well as to unravel the common complex genetic predisposition of these childhood epilepsies [Rudolf et al., 2009]. It also has important implications for the development of pharmaceutical agents that can target and suppress IEDs.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree