The VEP are useful for the assessment of the visual pathway. It can be especially useful in the young, nonverbal child when visual symptoms cannot be reported. The VEP are also used for objective evaluation of the visual system when standard vision testing cannot be performed due to young age or pathology. A flash or patterned stimulus can be used to elicit a VEP. A flash stimulus can be elicited with a light-emitting diode (LED) or a strobe light. A checkered pattern that reverses (or shifts) in color is used to elicit a patterned stimulus.
Maturational Issues
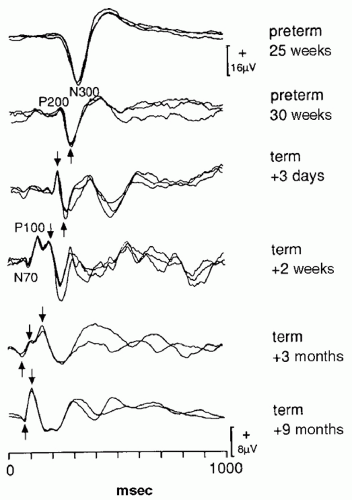
Flash and pattern reversal VEP (PRVEP) undergo maturational changes at different rates. Flash VEP can be recorded in infants as young as 22 weeks conceptional age as a single, large negative peak at about 300 msec (N300) (
5). Over the next several weeks, a positive peak appears before the P300. By term this positive peak becomes more prominent and occurs at about 200 msec (P200). The P200 becomes a bifid peak by 44 weeks gestational age. The earlier positive peak is referred to as the P100. Over the next several weeks the P100 shortens in latency, and by 6 months of age the P100 is the dominant waveform that has a latency of about 100 msec. Thereafter there are few changes in the flash VEP. The LED and stroboscopic VEP have minor maturational differences, but overall maturational changes are similar (
5) (
Fig. 49.1). Because of these slight differences, laboratories should obtain normative data with particular type of stimulus, and if normative data from another laboratory is used, the method of stimulation must be kept constant.
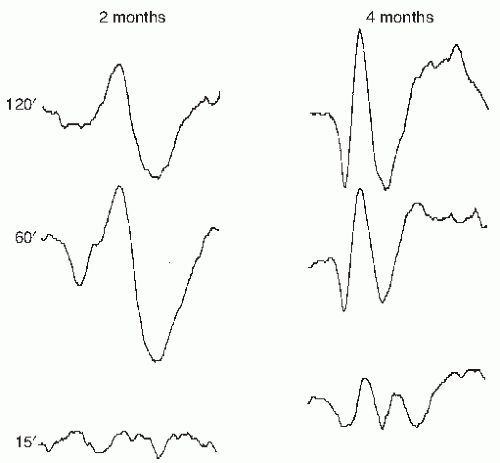
PRVEP can be obtained in very young children, although with more difficulty than flash VEP. Maturation of PRVEP is dependent on the size of the checks being used to elicit the responses. At birth, large check sizes (120-minute arc) produce a simple, positive peak at 200 msec (
5). By 2 months, negative waveforms appear before and after the large positivity. The latency of these peaks shorten by 10 msec per week until 5 months of age (
6). By 6 to 8 months of age, the PRVEP to large checks resembles that of an adult. PRVEP evoked with medium
(60 minutes) and small (15 minutes) checks have a slower maturation rate. PRVEP to small checks are not present at birth and appear by 6 months of age. They do not reach adult morphology and latency until after age of 5 years (
Fig. 49.2). Additionally, PRVEP recorded with binocular stimulation have a shorter latency than if recorded with monocular stimulation (
7).
Methodology
In the older child, routine methods for obtaining VEP as recommended by American Clinical Neurophysiology Society (ACNS) guidelines should be followed (
8). For infants and young children, several additional factors must be considered, including methodology of electrode application, type and method of stimulation, and recording techniques.
Electrodes should be applied with saline jelly and nonirritating tape in neonates (
5). Abrading should be kept to a minimum as skin is very sensitive. In older children, adhesive paste and gauze can be used. Collodion can also be used, but produces a strong odor that may be irritating to children. Electrodes are applied in MO (or Oz) and MF (Fz) locations at a minimum. The latter electrode serves as the reference. Reference electrode can also be put on the mastoids or earlobes bilaterally. Additional electrodes in the LO and RO locations can also be applied. The occipital electrodes are referenced to the MF electrode to create a one- to three-channel recording montage. In the very young child, a single-channel montage (MO-MF) may be preferred to minimize the number of electrodes applied, especially when flash VEP are being acquired. In the older child, a four-channel montage can be used, as shown in
Table 49.1A (
8). The montage used in the author’s laboratory is shown in
Table 49.1B; this montage allows better analysis of the topography of the P100 waveform.
Flash and PRVEP can be used in children. As noted above, a flash stimulus can be delivered through an LED goggles or strobe lamp. The former is preferred as it delivers the stimulus in a more focused and unilateral manner. Since normative values of VEP obtained to the different types of flash stimuli can be different, studies must be performed in the manner in which the normative data was obtained (
9). In term neonates and young children, flash VEP can be obtained in light sleep. While the latency is the same as when awake, the amplitude of the response may be smaller. In preterm infants, however, flash VEP are not reliably obtained if the child is asleep (
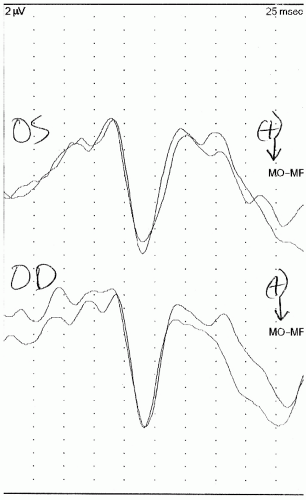
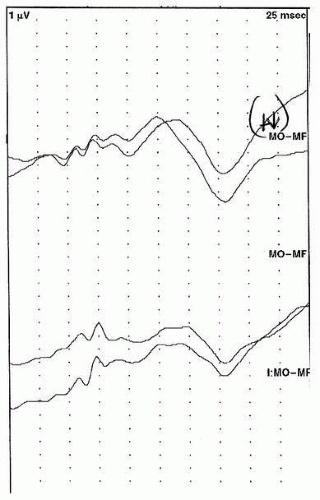
10). These children should be kept awake for the duration of testing. An example of a normal flash VEP is presented in
Figure 49.3.
PRVEP are obtained with checkerboard pattern generated on a computer monitor. The P100 latency can be affected by the type of computer monitor used, especially liquid crystal display (LCD) monitors (
11). The child should be alert and attentive to testing wearing corrective lenses, if necessary. Averaging should be paused if the child looks away from the stimulus. An artifact rejection paradigm should be used to eliminate epochs with movement artifact. Distractions should be minimized in the testing room. Cartoons superimposed on the checkerboard pattern have been used to help children focus; this results in lower P100 amplitude but does not affect latency (
12). PRVEP have been recorded in young children in light sleep (
13). The P100 amplitude is lower and latency prolonged in this situation. The optimal check size used for PRVEP depends on the age of the child. In the first 6 months of age, 120′ is most appropriate, at 2 months 60′, 3 to 5 months 30′, and thereafter 15′ (
7). If a response is not obtained, the check size should be doubled. In children younger than 6 months of age, binocular testing is performed. Thereafter, monocular testing can be performed. When monocular testing is performed, the child’s ability to cooperate is enhanced if the better eye is tested first (
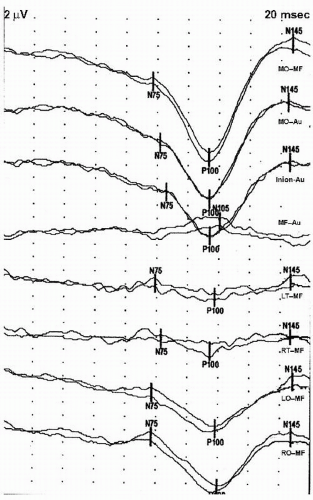
7). An example of a normal PRVEP is shown in
Figure 49.4.
Flash VEP are recorded with filter settings of 1 to 100 Hz. In premature infants 20 to 30 repetitions are needed to obtain a reproducible response. Older children have a slightly lower amplitude response and require 50 to 60 repetitions. The stimulus rate in children less than 1 year of age should be 0.5 per second with a 1000-msec sweep, and after 1 year a rate of 1.9 per second and a sweep of 500 milliseconds are recommended (
5).
Filter settings for PRVEP are usually 1 to 100 Hz; however, some laboratories use 30 or 50 Hz for the high-frequency filter. In neonates and very young children, 30 to 50 repetitions may be sufficient to obtain a reproducible response. In older children, 50 to 100 repetitions are necessary. The checkerboard pattern is presented at a rate of 1.9 reversals per second, and a sweep of 500 milliseconds is used (
5).
Interpretation
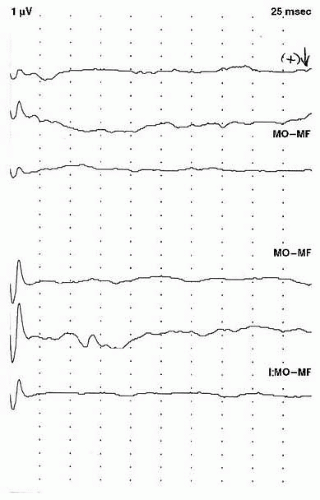
The main waveform of interest in VEP testing is the P100, which is a positive peak occurring approximately 100 milliseconds after stimulation. When the VEP is obtained with a flash stimulus, the P100 latency and amplitude have high interindividual variability. This makes using these parameters to determine normalcy difficult. If latency or amplitude is used to denote an abnormality, caution must be exercised to ensure these values are well beyond the excepted upper limit of normal for that laboratory (
Fig. 49.5). Because of the variability of the P100 obtained with flash stimulation, many laboratories
consider the presence of a P100 response at any latency as normal, and an abnormal study is one in which there is no reproducible waveform (
8) (
Fig. 49.6).
The presence of a flash VEP implies that at least some stimuli are reaching the visual cortex from the retina. Whether the macula or peripheral retina is being activated to produce the flash VEP cannot be determined. Moreover, the presence of a normal flash VEP does not imply conscious visual perception (
8). When the flash VEP is absent (abnormal), an electroretinogram (ERG) should be obtained. A normal ERG with absent flash VEP suggests a lesion in the visual pathway, whereas an absent ERG implies a lesion in the retina and possibly also in the visual pathway (
8).
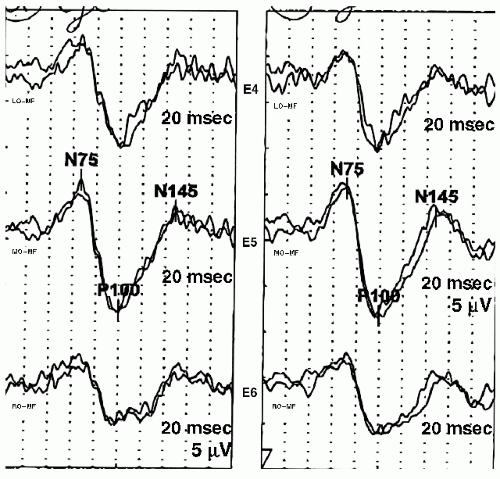
The P100 obtained with pattern reversal stimulation has little interindividual variability within a particular age group. Latency measurements can be used to determine normalcy. Amplitude, however, continues to be variable, and mild amplitude asymmetry between the two eyes is not a reliable indicator of pathology. The P100 latency should be compared to laboratory age-specific norms. Published normative data for PRVEP P100 latency are reproduced in
Table 49.2 (
14). This data has normal latencies of the P100 for various age groups, monocular or binocular stimulation, and a variety of check sizes. If published normative data are used, stimulation and recording paradigms should mirror those that were used to acquire this normative data. An example of a PRVEP with prolonged P100 latencies is shown in
Figure 49.7.
Clinical Utility
VEP have been used in children to evaluate many different issues ranging from vision to prognosis in high-risk infants. In the very young child, flash VEP have to be used, but in older children PRVEP can provide much more data. The common uses of VEP are discussed below.
Vision Testing
There are several ways of assessing visual acuity in preverbal children. Although several ophthalmologic techniques are available, PRVEP can also be used in children as young as 3 months. The PRVEP have been shown to have good correlation with ophthalmologic methods of visual acuity estimation (
15). A reproducible PRVEP obtained at 15-minute check size corresponds to a normal visual acuity (
16). By 6 months infants should have the equivalent of 20/20 visual acuity (
7).
VEP can be useful in determining the site of dysfunction in children with impaired vision. Performing PRVEP with an ERG in this situation can localize the problem to the retina if the ERG is abnormal or to the visual pathway if the PRVEP are abnormal and ERG is normal (
7). However, similar findings (normal ERG and abnormal PRVEP) can also be noted in children with developmental delay. Developmental delay can be differentiated from vision loss by performing serial VEP. A gradual improvement of VEP is consistent with delayed maturation.
Flash VEP have been used to predict outcome in children with acute vision loss from a variety of causes. When flash VEP are performed during the period of blindness and normal responses are noted, favorable visual outcome is likely. Visual improvement, however, can be delayed for a few years. If the VEP are absent, blindness most likely will persist (
17).
Perinatal Injury
Many types of perinatal injury can lead to permanent neurologic complications. Various neurophysiologic tests have been
used to help predict which high-risk newborns are likely to have persistent neurologic deficits. VEP have been used to evaluate several types of perinatal injuries as clinical examination of these children can be challenging and ancillary tests can add useful additional information.
Perinatal Asphyxia
With improved critical care survival rates of newborns suffering from perinatal asphyxia have increased. Flash VEP and other neurophysiologic tests have been used to predict outcomes in these children and provide guidance for further supportive care. Several studies have shown that absent flash VEP predict severe neurologic disability (
18,
19 and
20). Many children with abnormal VEP expectedly had visual system problems. Normal flash VEP are likely to be associated with good outcomes (
21). In most studies, VEP were performed within the first week of injury.
Intraventricular Hemorrhage and Periventricular Leukomalacia
Inconsistent flash VEP findings have been reported in children with intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL). Several studies have noted absence or severe delay of the main positive waveform of the VEP (
22,
23,
24 and
25). Others have reported these abnormalities to only inconsistently be associated with IVH and PVL (
26,
27). Generally, however, repeat testing in patients with IVH usually demonstrates VEP abnormalities (
28).
Preterm Infants
Flash VEP have been used to predict prognosis of preterm infants with inconsistent results. Some authors report that flash VEP do not help predict outcome of children up to 35 weeks of
gestation (
29,
30). Others have noted the utility of VEP in this situation. Abnormal flash VEP have been reported to have a 89% to 97% specificity for death or cerebral palsy (
28,
31,
32). Additionally, normal flash VEP have been reported to have a specificity of 94% for favorable outcome (
28).
Developmental Delay
Children with spastic diplegic or hemiplegic cerebral palsy have been evaluated with PRVEP. The P100 latencies are longer in children with cerebral palsy than those in normal controls. However, the extent of prolongation does not correlate with the severity of cerebral palsy or mental retardation (
33). So far VEP have demonstrated only marginal utility in the evaluation of children with developmental delay and cerebral palsy.
Demyelinating Diseases
Although less common than in adults, demyelinating diseases can occur in children. PRVEP can be very helpful in detecting clinically evident or silent lesions. In cases of optic neuritis, VEP are abnormal, showing either loss or prolongation of the P100 (
5). With clinical improvement, VEP also improve. However, even after complete clinical recovery, the P100 latencies remain prolonged beyond normal (
34). Patients with multiple sclerosis are also very likely to have abnormal VEP even if they do not have visual symptoms (
5,
7).
Hydrocephalus
Studies evaluating the utility of VEP in very young children with hydrocephalus have found prolongation of the P100 (
35,
36). After shunting, the P100 improves. Subsequent deterioration of hydrocephalus results in prolongation of the P100. If complications occur during shunt placement, the improvement of the P100 is not seen. In older children with hydrocephalus, the P100 prolongation is also noted; however, improvement is not consistently seen with shunting (
5). Thus, while VEP can be helpful in diagnosis of hydrocephalus, they cannot be used to follow the disease course.
Neurodegenerative Diseases
Various types of leukodystrophies, metabolic disorders, and hereditary ataxias have been evaluated with VEP.
Leukodystrophies and Other Metabolic Disorders
Leukodystrophies are a group of inherited white matter diseases that can present from early childhood to adulthood. Genetic testing can definitively establish the diagnosis in many of these conditions and can even lead to prenatal diagnosis in the high-risk fetus. Neurophysiologic tests can help determine which parts of the nervous system are most affected and may be useful in monitoring the progression and treatment of these conditions.
Children with adrenoleukodystrophy, metachromatic leukodystrophy, and Pelizaeus-Merzbacher disease have been studied with VEP. In all these disorders, VEP have been found to be abnormal with the exception of one patient in the early stages of adrenoleukodystrophy (
37,
38). Patients with early infantile Krabbe disease were noted to have abnormal flash VEP in about 50% of cases, whereas most patients with late-onset Krabbe disease had a normal VEP (
39). As the disease progresses, VEP are likely to become abnormal. Absence of flash VEP also correlated with more severe changes noted on magnetic resonance imaging (MRI) (
40). Children with mucopolysaccharidosis III have also been studied with flash VEP. Early in the course of this disease, VEP are usually normal (
41). In several of these metabolic disorders, visual pathways do not appear to be involved early in the disease course but do become affected with disease progression.
Although a biopsy is needed for definitive diagnosis of ceroid lipofuscinosis, VEP and ERG can be helpful in selecting which patients to send for biopsy. The ERG is always absent in this condition, and the VEP often shows a prolonged latency and low amplitude. The exception occurs in patients with the late infantile variety of ceroid lipofuscinosis, in which case the VEP amplitude may be high (
42). In children with normal ERG and VEP, other diagnoses should be considered.
Hereditary Ataxias
Various hereditary ataxias have been noted to have VEP abnormalities. Patients with ataxia telangiectasia have low amplitude or absent P100 responses. In Friedreich ataxia, the VEP has a prolonged latency and high amplitude (
43). These findings, however, are not sensitive enough to use as the differentiating criteria between these disorders.
Neurofibromatosis
Neurofibromatosis is often associated with optic nerve gliomas. Several studies have evaluated the utility of VEP in patients with neurofibromatosis. In patients with optic nerve gliomas that affect visual acuity, VEP are abnormally prolonged (
44). Even neurofibromatosis patients with optic nerve gliomas and no visual symptoms have a high likelihood of having an abnormally prolonged P100 latency (
45). Additionally, patients without an identifiable lesion of the visual pathways can also have abnormal VEP (
46). An abnormal VEP study in a patient with neurofibromatosis, regardless of whether visual symptoms are present, should result in visual pathway imaging.
Coma
The utility of almost every neurophysiologic test has been analyzed in coma; flash VEP are no exception. SEP are more reliable predictors of coma outcome than VEP; however, since VEP also test the cortex, they can be used if SEP are unavailable. Absent flash VEP predicted poor outcome with an accuracy of 76% and a specificity of 92% (
5,
47). However, normal flash VEP predicted a normal outcome only 60% of the time. When an abnormal VEP is noted within the first few days of coma in a young child, it should be repeated as these tests can change considerably over a few days in this age group (
7).
Monitoring Visual Pathways
Many types of therapies may have toxic effects on the visual pathways. These complications of therapy may not be reliably reported by young children. VEP have been used to monitor potentially toxic therapies to detect subtle changes that may lead to alteration of management. One such application may be chelation therapy with deferoxamine for chronic inherited anemias. Deferoxamine has neurotoxic effects on the nervous system. VEP changes are a sensitive marker for these toxic effects and may be used to monitor treatment (
48).
Vigabatrin is an antiepileptic medication with an unusual side effect of visual field constriction. In order to be treated with this medication, patients must undergo serial visual field testing. Field-specific PRVEP have been shown to have a high sensitivity and specificity in identifying vigabatrin-induced visual field defects (
49).
VEP have been used to monitor chemotherapy toxicity in children with leukemia (
50). Others have used VEP to monitor the effects of ethambutal in children with tuberculosis (
51). These studies demonstrate some utility of VEP in detecting early neurotoxicity.
Miscellaneous
Children with migraines have been evaluated with PRVEP. The P100 latency is longer and amplitude higher in patients with migraines (
52,
53). Among the children with migraines, those with aura tend to have lower P100 amplitudes compared to children with migraine without aura and migraine variants (
52).
PRVEP testing has been performed in children and their relatives with Wilson disease. The P100 is prolonged in about 25% of patients with neurologic symptoms and 11% of patients without neurologic symptoms (
54). Presymptomatic siblings of children with Wilson disease may also have VEP abnormalities. These abnormalities occur earlier than Kayser-Fleischer rings, allowing earlier decisions regarding treatment (
55). Children who are candidates for liver transplantation have also been studied with VEP. These children were studied before the occurrence of clinical hepatic encephalopathy, and they were noted to have a prolonged N75 latency (
56). These findings may suggest early involvement of the nervous system
in hepatic disease, possibly increasing the urgency of transplantation.
Patients with albinism have unusual projections of their visual pathways. Not only the nasal but also the temporal retinal fibers project to the contralateral side. This can be detected with both flash VEP and PRVEP. Flash stimulation results in higher amplitude VEP recorded over the contralateral occipital cortex (
57). Full-field PRVEP results in shorter latency P100 response over the contralateral hemisphere (
58,
59). These findings can help distinguish patients with albinism from other clinically similar conditions such as congenital cone dysfunction and idiopathic nystagmus (
58).