Nature, indeed has had a triple end in view in the distribution of nerves: she wished to give sensibility to organs of perception, movement to organs of locomotion, and to all others the faculty of recognizing the experience of injury.
I. INTRODUCTION TO TESTING GENERAL SOMATIC SENSATIONS
A. Special and general senses
The special senses consist of sight, smell, taste, hearing, and equilibrium. The general senses tested in the Standard neurological examination (NE) consist of touch, pain, temperature, position, vibration, and stereognosis. Unique receptors and unique central pathways mediate each of the special senses. Some skin receptors and somatosensory pathways serve one general sensory modality, but other receptors in the skin are polymodal.
B. Negative and positive sensory phenomena after lesions of central and peripheral sensory pathways
1. Abnormal or noxious sensations like pain may arise in two ways: from stimulation of receptors or from intrinsic disease of the nerves or central pathways. Disease of afferent nerve fibers centrally or peripherally causes not only negative or deficit phenomena, with lack of sensation, as you would expect, but also, paradoxically, may cause positive phenomena, with excessive sensation, that is, pain and tingling (Bouhassira and Attal, 2011; McArthur, 2012).
2. Nomenclature for deficits in superficial sensation
a. Esthesia = touch or feeling, so hypesthesia = partial loss of touch, and anesthesia = total loss. Anesthesia is also used to mean lack of pain.
b. Therm = heat, so thermhypesthesia = partial loss of temperature sensation, and thermanesthesia = total loss.
c. Algesia = pain, so hypalgesia = partial loss of pain sensation, and analgesia (or anesthesia) = total loss.
3. Nomenclature for pain and other noxious irritative phenomena after disease of peripheral or central sensory pathways
a. Hyperesthesia, hyperalgesia, and hyperthermesthesia refer to an increased sensitivity to touch, pain, and temperature, respectively. For example, after a burn of an area of skin, even slight exposure to heat may cause intense pain, that is, hyperthermesthesia.
b. Paresthesias and dysesthesias are uncomfortable sensations of numbness, tingling, pins and needles, or burning pain short of neuralgia or causalgia. Paresthesias describes such sensations when they accompany a normal external stimulus to the skin. Dysesthesias describes their spontaneous occurrence without any obvious external stimulus or during movement, pressure or touching. You will notice paresthesias and dysesthesias if you study your own sensations when recovering from a local anesthetic for a dental procedure or after having sat too hard on your own sciatic nerve, causing it to “go to sleep.”
c. Hyperpathia means an extreme overresponse to pain. Hyperpathia associated with a raised pain threshold is called anesthesia dolorosa (represents a deafferentation pain that is felt in an area that is otherwise numb, and now most commonly seen following surgery for trigeminal neuralgia).
d. Neuralgia means multiple, very severe, electric shock-like pains that radiate into a specific root or nerve distribution. Examples include trigeminal neuralgia and postherpetic neuralgia that commonly follow herpetic infection of a dorsal root ganglion (Johnson and Rice, 2014). The neuralgias may or may not alter the sensory threshold, but they complicate testing because of the intense pain and the presence of trigger points that, if touched, elicit a jolt of pain.
e. Causalgia, named by S. Weir Mitchell (1829–1914) but now called Complex regional pain syndrome (CRPS; CRPS Type 1 includes reflex sympathetic dystrophy and other disorders not preceded by a nerve injury while CRPS Type II follow damage to a peripheral nerve), designates an unbearable, burning, relentless hyperesthesia and hyperalgesia that ensue after injury to a peripheral nerve (Borchers and Gershwin, 2014; Birklein et al, 2015). Merely a puff of air or slight movement triggers unbearable pain.
f. Special irritative or positive sensory disorders of cranial nerves: See Section II C.
g. Neurophysiologic mechanisms of such hyperesthesias might include:
i. Excessive firing of diseased neurons because of destabilization of their membranes.
ii. Changes in the sensitivity of receptors or of central conducting mechanisms (Bennett and Woods, 2014).
iii. A discrepancy or imbalance of the sensory information conveyed when disease affects the ratio of impulses delivered by fibers of different diameters.
iv. Cross talk or short circuiting of impulses in demyelinated axons.
II. GENERAL ANATOMICO-PHYSIOLOGIC PRINCIPLES IN ANALYZING LESIONS OF THE PERIPHERAL NERVOUS SYSTEM
A. Principle 1: The signs and symptoms of peripheral nervous system (PNS) lesions will be expressed in the anatomic distribution of roots, nerve trunks, plexuses, or peripheral nerves
B. Principle 2: The signs and symptoms of PNS lesions depend on the functional types of axons in the nerve: motor (general somatic and visceral efferent, branchial efferent), sensory (general somatic and visceral afferent, special visceral and somatic afferent, or mixed sensorimotor, visceral, and somatic)
C. Principle 3: The signs and symptoms of PNS lesions depend on whether the lesions cause deficit phenomena, irritative phenomena, or both
1. Deficit phenomena after lesions of the somatomotor axons (general somatic efferent, branchial efferent):
a. Paresis or paralysis of individual muscles.
b. Decreased or absent muscle stretch reflexes (MSRs).
c. Denervation atrophy (early and severe).
2. Release phenomena after lesions of somatomotor neurons:
a. Fasciculations.
b. Fibrillations.
3. Autonomic deficit phenomena after lesions of visceromotor neurons (general visceral efferent):
a. Paralysis and atony of smooth muscle, impairing peristalsis, propulsion, and emptying.
b. Vasomotor paralysis with vasodilation, orthostatic hypotension, and impotence.
c. Anhidrosis.
d. Trophic changes consisting of hair loss, atrophy of skin, and dystrophy of nails.
4. Autonomic release phenomena after lesions of the visceral motor neurons (general visceral efferent)
a. Hyperhidrosis.
b. Vasomotor instability with vasoconstriction and hyperhidrosis, rather than anhidrosis and vasodilation; often Raynaud phenomenon.
5. Deficit phenomena after lesions of the somatosensory axons (general somatic afferent)
a. Anesthesia or hypesthesia.
b. Analgesia or hypalgesia.
c. Thermanesthesia or thermhypesthesia.
d. Loss of dorsal column modalities.
6. Irritative phenomena after lesions of the somatosensory or special sensory axons or, in some cases, their central pathways
a. Hyperesthesias: paresthesias and dysesthesias
b. Hyperalgesia
c. Hyperthermesthesia
d. Hyperpathia (anesthesia dolorosa)
e. Neuralgia
f. Complex regional pain syndrome Type II
g. Symptoms specific to irritation of sensory cranial nerves
i. Cranial nerve (CrN) I: hyperosmia (usually central in origin).
ii. CrN II: flashes of light, scintillating scotomas (phosphenes or “seeing stars”).
iii. CrNs V and IX: trigeminal and glossopharyngeal neuralgia.
iv. CrN VII: hypergeusia (increased sense of taste, usually central lesion).
v. CrN VIII: tinnitus, hyperacusis, diplacusis (auditory division), and vertigo (vestibular division).
D. Principle 4: The sensory nerves function according to the doctrine of specific nerve energies of Johannes Muller (see page 339)
E. Principle 5: To localize lesions of the PNS to a root, plexus, peripheral nerve or branch, detect and list the weak muscles and outline with ink the area of sensory deficit on the patient’s (Pt’s) skin or on a diagram. Then compare the results with charts of dermatomal, myotomal, and peripheral nerve distributions (Chapter 2)
F. Principle 6: For the localizing analysis, “think along” the course of the PNS nerve fibers, in the direction of the nerve impulse
1. To analyze a sensory disorder in the PNS, commence with the skin and “think along” the PNS from the receptor through the nerve branch or trunk, plexus, and dorsal root into the spinal cord. Continue thinking along the pathway to the primary receptive cortex.
2. To analyze a motor disorder, start with the motor cortex and think through the pyramidal tract, ventral motoneuron, ventral root, plexus, nerve trunk, neuromyal junction, and the muscle fiber itself.
G. Principle 7: The size of the nerve fiber and its myelination determine its conduction velocity and relates to its function. Measurement of sensory or motor nerve conduction velocity is often an important adjunct to the clinical examination
1. Peripheral nerves contain unmyelinated and myelinated nerve fibers ranging in diameter from 0.2 to 20 μm (myelin sheath included).
a. The unmyelinated axons are around 1 μm in diameter (a red blood cell is 7 μm) and conduct at about 1 m/s. They range in size from approximately 0.2 to 0.3 μm to approximately 3 μm.
b. The myelinated fibers range from 1 to 2 μm to 20 μm in diameter and conduct at rates up to 120 m/s, with a mean near 60 m/s.
60 m/s × 60 s/min × 60 min/h × 1/mile 1609.3 m
= 216 000/1609.3, or 134 miles/h
2. In general, the larger the fiber diameter, the longer the internodal distance and the faster the conduction. Those fibers conducting faster than 3 m/s are myelinated.
3. The largest nerve fibers innervate joint receptors and muscle fibers.
4. Purely cutaneous peripheral nerves, such as the sural nerve (often chosen for biopsy) or the superficial radial nerve, contain no afferents from joints or muscles and generally have fibers smaller than 12 μm in diameter.
5. In mixed nerves the smallest fibers, generally unmyelinated, are postganglionic sympathetic efferents, visceral afferents, or serve as pain and temperature afferents. The small fibers of purely cutaneous nerves contain only the latter.
6. To classify nerve fibers by size, physiologists use a confusing mixture of three systems: English alphabets (A, B, and C), Greek alphabets (a, b, g, and d), and Roman numerals (I to IV) (Menorca et al, 2013).
III. GENERAL CLINICAL PRINCIPLES IN TESTING ALL SOMATIC SENSATIONS
A. For the patient
1. To enliven the proceedings, make each test a game or a matter of curiosity: “Let us see how light a touch you can feel.” “Let us see whether you can feel the buzz of the tuning fork.”
2. Demonstrate and describe the tests. Ask for yes or no responses, or “Is (stimulus) one different from (stimulus) two?” a procedure called forced-choice testing.
3. Have the Pt close the eyes to avoid visual cues.
B. For the examiner
1. Compare homologous areas of the right and left sides and compare normal areas to any suspected abnormal areas.
2. The skin areas differ greatly in sensitivity. The highly sensitive skin of the face and armpits contrasts with the horny skin of palms and soles. Hairy skin perceives tickling and touch better than glabrous skin. The forehead is the most sensitive area for temperature discrimination. Because cold skin loses sensitivity, ensure a warm skin before testing the Pt.
3. Plan follow-up examinations to recheck any doubtful results.
4. Determine whether sensory deficits match a central pathway, segmental (dermatomal), plexus, or peripheral nerve pattern (Figs. 2-10 and 2-11) or match a nonanatomical distribution (Chapter 14).
5. Recognize that the Pt’s mental state, legal issues, or somatization from the illness may drastically alter sensory test results.
BIBLIOGRAPHY · Introduction to Testing General Somatic Sensations
Bennett DLH, Woods CG. Painful and painless channelopathies. Lancet Neurol. 2014;13:587–599.
Birklein F, O’Neill D, Schlereth T. Complex regional pain syndrome: an optimistic review. Neurology. 2015;84:89–96.
Borchers AT, Gershwin ME. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13:242–265.
Bouhassira D, Attal N. Diagnosis and assessment of neopathic pain: the saga of clinical tools. Pain. 2011;152:S74–S83.
Johnson RW, Rice ASC. Postherpetic neuralgia. N Engl J Med. 2014;371:1526–1533.
McArthur JC. Painful small fiber neuropathies. Continuum Lifelong Learning Neurol. 2012;18:106–125.
Menorca RMG, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29:317–330.
IV. EXAMINATION OF SENSORY FUNCTIONS OF CRANIAL NERVE V
A. Functions of the afferents of cranial nerve V
1. Sensory domain of the trigeminal nerve: With a single scimitar blow, let us slice the face off from the head, along the line shown in Fig. 10-1, creating the “Mask of Trigeminus.”
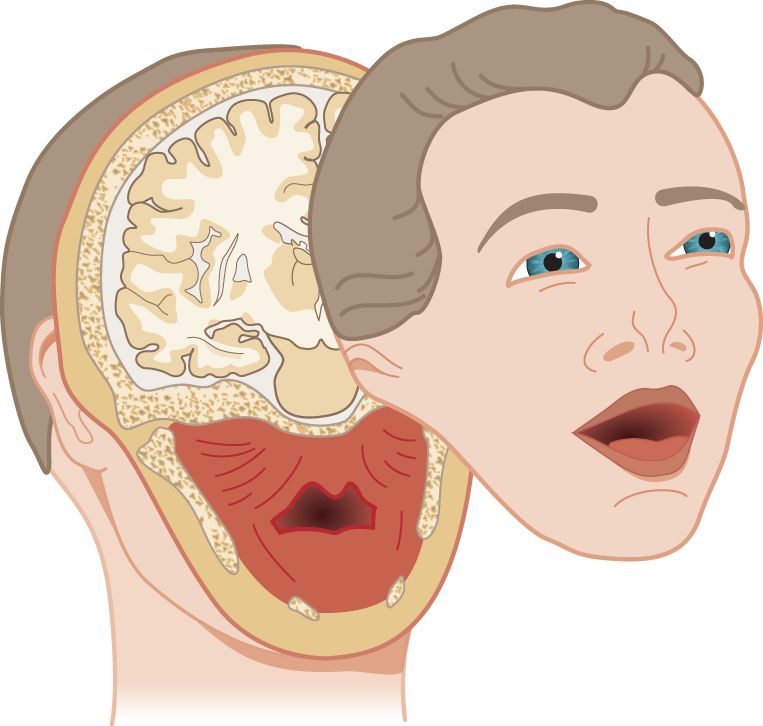
FIGURE 10-1. The “Mask of Trigeminus.” Obtain it by a single slice through the head with a scimitar.
2. The three dimensions of the Mask of Trigeminus
a. The mask sliced away by the scimitar is no ordinary Halloween mask. It has three dimensions. It contains all the territory that receives motor and sensory innervations from CrN V: chewing muscles and their proprioceptors, proprioceptors from the eye muscles and general sensation from the facial skin, eyeball and orbit, mucous membranes of the nose, mouth, tongue, and sinuses, the dura mater, and a tad of the external ear (Siemionow et al, 2011) (Video 10-1).

Video 10-1. Trigeminal trophic syndrome.
b. Of the tissues in the slice, only the cerebrum has no nerve supply. CrN V conveys no special sensory fibers. Notice that the cut spares the angle of the mandible, leaving it behind with the head. See the mandibular angle in Fig. 10-2.
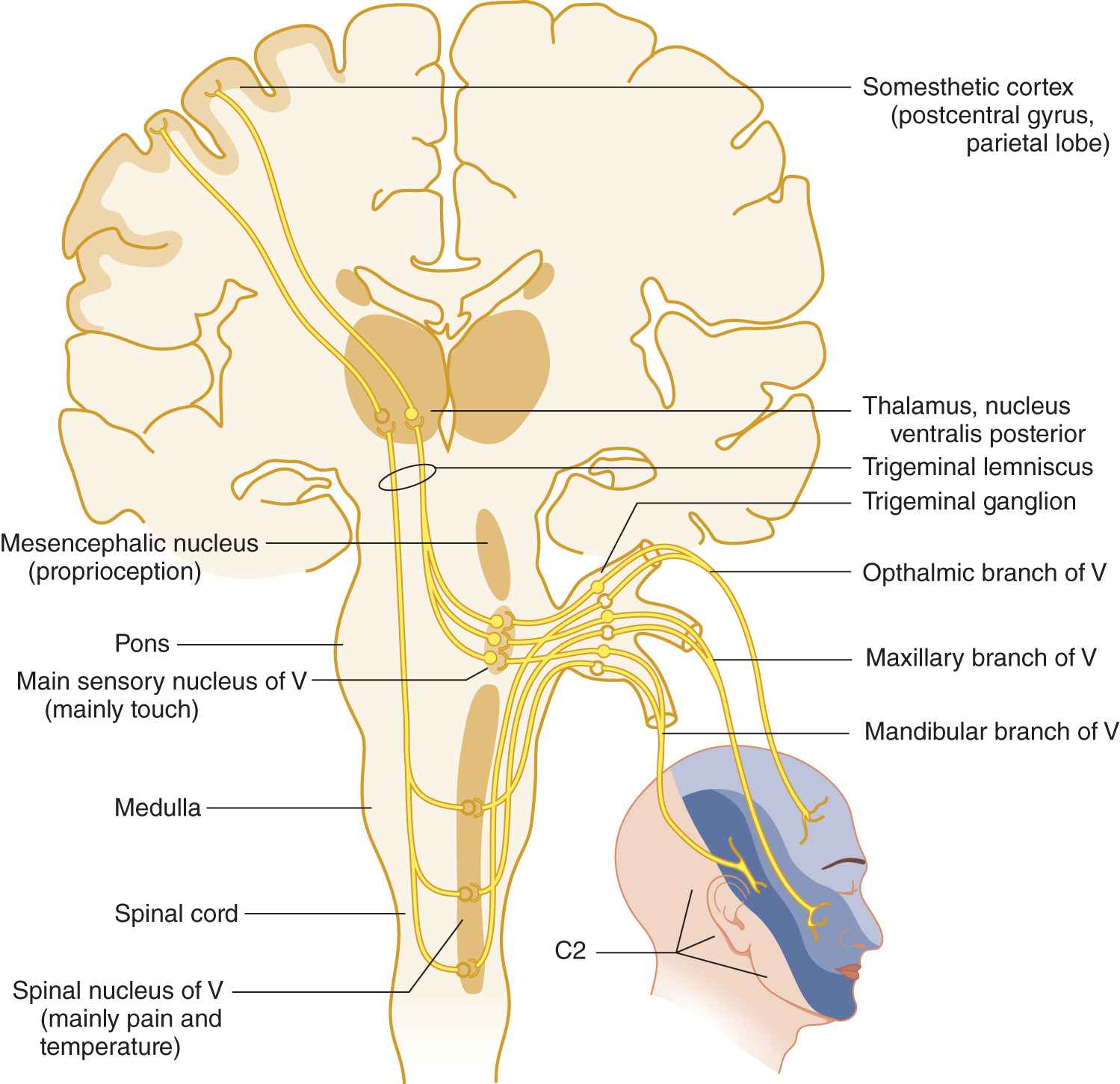
FIGURE 10-2. Peripheral and central connections of the trigeminal nerve. Notice the pain and temperature pathway descending to the spinal cord. Compare the posterior margin of the line of facial innervation with that shown in Fig. 10-1.
3. How to best appreciate (and remember) the amazing variety of functions of afferents from CrN V
a. First, observe a newborn baby: It roots for a nipple, sucks, chews, swallows, searches with its tongue, blinks, cries, and sneezes. Stimuli acting through CrN V initiate all of these reflexes. Then, too, you will understand that the turning of the head toward the stimulus in the rooting reflex depends on the descending root of CrN V that connects with the head-turning muscles of the neck. In fact, the very first cutaneous reflex that the human fetus exhibits is turning of the head in response to stimulation of the upper lip and appears when the fetus reaches week 8 of gestation, just after the axons of the root of CrN V have grown down into the cervical region to make this reflex possible (Hooker, 1969). The anatomist Davenport Hooker PhD, between 1932 and 1963, performed and filmed noninvasive studies of reflexive movement on surgically aborted human fetuses from which this statement is based (Wilson, 2014); anatomical pathways for pain are developed and functional in the human fetus by 26 weeks of gestational age, but it is not clear if the perception and behavioral aspects of pain are equally functional (Derbyshire, 2006). To further understand the descending connections of CrN V, consider the rat, a nocturnal animal with weak eyes, as it feels its way along a totally dark passage. By touching its whiskers against the wall, it gains afferent information to orient the head, neck, and body.
b. Next consider the autonomic responses mediated by CrN V: lacrimation from corneal irritation, rhinorrhea, salivation from mechanical stimulation of the oral mucosa (as opposed to taste), and pupillodilation and bradycardia or tachycardia from facial pain.
c. Then, think of the role of CrN V in sinus pain and toothache, not to mention headache in general. Sensory V is a busy nerve, and that is why it has the largest sensory ganglion in the body.
B. Distribution of the sensory divisions of cranial nerve V
1. From Fig. 10-2 learn the three divisions of the trigeminal nerve, their facial skin areas, and central connections.
2. CrN V receives its name trigeminal because of its three large sensory divisions, the _________
3. The three sensory branches funnel into a single root that attaches to the  mesencephalon/
mesencephalon/ pons/
pons/ medulla. (
medulla. ( pons)
pons)
4. The sensory ganglion of CrN V, the trigeminal (semilunar, gasserian) ganglion, contains the  primary/
primary/ secondary/
secondary/ tertiary neuron of the sensory pathway from the face. (
tertiary neuron of the sensory pathway from the face. ( primary)
primary)
5. The trigeminal ganglion corresponds to the _________
6. The primary ganglia for the special senses that are very near their end organs belong to CrNs _________
_________
7. The trigeminal nuclei (nuclei, not ganglia) contain the  primary/
primary/ secondary/
secondary/ tertiary neuron of the trigeminal pathway. (
tertiary neuron of the trigeminal pathway. ( secondary)
secondary)
8. Where is the tertiary neuron? _________
9. The mesencephalic nucleus of CrN V violates the rule that the primary neuron of a sensory pathway is outside the central nervous system. This nucleus consists of primary neurons within the neuraxis, a unique fact but of no clinical use. The mesencephalic portion of CrN V probably mediates proprioception; the pontine and rostral medullary portion mediates touch; and the spinal nucleus mediates pain and temperature. Thus, in rostrocaudal order, the functions mediated by the three portions of the sensory nucleus of CrN V are _________
10. Taken together, trigeminal sensory fibers and nuclei extend from the rostral part of the cervical region of the spinal cord to the mesencephalon (Fig. 10-2).
C. Technique for testing touch in the area of cranial nerve V
1. Instruction to the Pt. Ask the Pt to say touch in response to each touch by a wisp of cotton. After the Pt closes the eyes, lightly brush each area of the three sensory divisions of CrN V with a wisp of cotton. Touch alternate areas and sides of the face randomly. Also, change the time between touches to keep the Pt from getting into a rhythm of answering without actually attending to the stimulus.
2. If you used a rigid test object to test for light touch, which principle of sensory testing did you violate?
_________
_________
3. After the Pt has responded several times, how would you test the Pt’s reliability and attentiveness?
_________
_________
4. As a rule, if the history indicates a specific area of sensory loss, start sensory testing in a normal area, to give the Pt the experience of the normal sensation. Then start in the middle of the abnormal area and work outward (Video 10-2).

Video 10-2. Pain in the V1 distribution in a patient with history of zoster ophthalmicus.
D. The corneal reflex
1. Anatomy of the corneal reflex arc
a. The corneal (or blink) reflex consists of closure of both eyelids in response to touching one cornea. It is entirely distinct from the corneal light reflection described earlier.
b. The afferent arc of the corneal reflex travels through the _________
c. The muscle that closes the eyelid is the _________
d. The corneal reflex thus tests the integrity of two cranial nerves, (afferent) _________
2. Technique for the corneal reflex
a. Use a free piece of cotton, rolled to a fine point. Do not use cotton attached to a stick (Q tip). Avoid sticks around the eyes. A Pt who has an intellectual disability, is demented, or delirious may flinch against the tip of the stick and injure their eye.
b. Tell the Pt, “I am going to just touch your eyeball very lightly.” Instruct the Pt to look to one side and a little up. Gently hold the lids apart to avoid stimulating the eyelashes.
c. Bring in a wisp of cotton from the lateral side to touch the lateral side of the cornea of the adducted eye (or adjacent sclera and then sliding it over to the cornea). Bring the cotton directly in from the side to avoid entering the field of vision. That would cause a visually mediated flinch, not a corneal blink reflex. In Fig. 10-3, make an X on the exact spot to stimulate the cornea without entering the Pt’s field of vision.
FIGURE 10-3. Blank to mark the site for stimulation of the cornea to elicit the corneal reflex.

3. Clinical interpretation of the corneal reflex: Most normal individuals display a corneal reflex. There are exceptions that include some elderly, postcataract or refractive vision correction surgery and other medical disorders that affect the cornea directly or the corneal nerves that innervate it (Shaheen et al, 2014). If no response occurs, do not conclude that the arc from CrN V to VII is interrupted until you have checked for a contact lens! Acute deep hemispheric parietal lesions may abolish the corneal reflex on the contralateral side for hours or days as a deficit or “shock” phenomenon. The corneal reflex behaves like other superficial reflexes, the abdominal and cremasteric reflexes, and they all temporarily disappear after an acute upper motor neuron (UMN) lesion. Further assessment of the pathway for this reflex can be accomplished through neurophysiological testing (Valls-Sole, 2012). As with the gag reflex, in the absence of symptoms, the Ex may elect to eliminate the corneal reflex from the screening NE.
E. The corneomandibular reflex (von Sölder phenomenon or Wartenberg winking jaw or reverse Marcus Gunn phenomenon)
Stimulation of one cornea causes contraction of the ipsilateral lateral pterygoid muscle and a deviation of the jaw to the opposite side and a developmental reflex not present after infancy. The jaw twitches contralaterally after stimulation of the cornea ipsilateral to a hemispheric lesion. A bilateral reflex may occur in coma, multiple sclerosis, or bilateral hemispheric lesions (Guberman, 1982). It may indicate a UMN lesion more sensitively in amyotrophic lateral sclerosis than other UMN signs and may also appear in parkinsonism (Okuda et al, 1999; Okuda et al, 2008).
F. The glabellar tap or blink reflex
This developmental reflex disappears in infancy and consists of an inability to suppress bilateral contraction of the orbicularis oculi muscles in response to light tapping of the glabella with the fingertip (or gently with a reflex hammer). It disappears unilaterally in acute hemiplegia and bilaterally in deep coma. Usually, it fatigues by about 10 taps, but in dementias and parkinsonism (Parkinson disease, progressive supranuclear palsy, and multiple system atrophy) it persists (disinhibited) and referred to as Myerson sign or Glabellar tap sign (Brodsky et al, 2004).
G. Technique for testing temperature discrimination
1. Instruction to the Pt: State, “I want to see how well you can tell warm from cool. Please close your eyes, and I will place something on your cheek. Tell me whether it is warm or cool.”
2. Tuning fork or finger test: Apply the metal shaft of a tuning fork to the side of the Pt’s cheek for a few seconds and then remove it and apply the side of your little finger to the same spot. Ask the Pt whether each is cool or warm (Fig. 10-4).
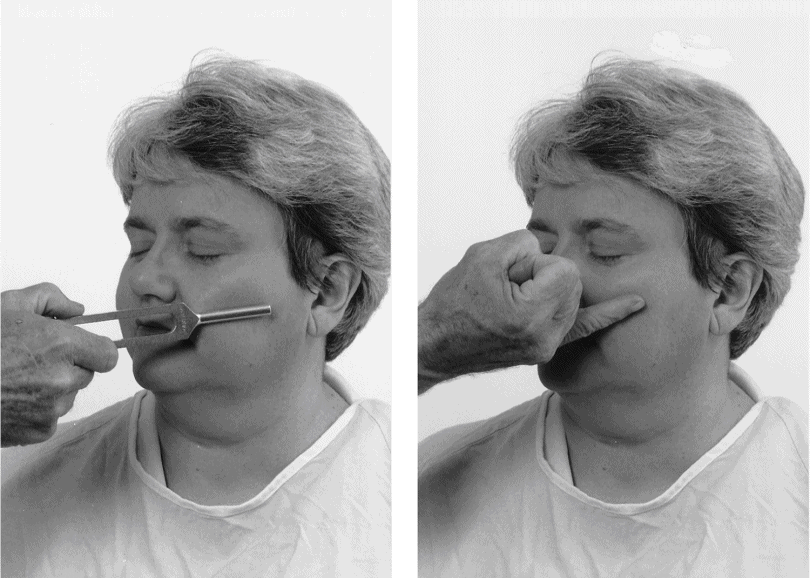
FIGURE 10-4. Randomly touch the tuning fork shaft and your little finger to test temperature discrimination.
a. Use the fork first to establish immediate communication because it will feel colder than the finger. The Pt will attend to the temperature and not to texture or size.
b. Randomly alternate the finger and tuning fork as you proceed over the three trigeminal sensory areas and then over the dorsum of both hands and both feet. Try this test on yourself.
c. To sharpen ambiguous results, say “Which is cooler, number 1 (the tuning fork) or number 2 (the finger)?”
3. Warm and cold tube test: Fill one tube with warm water and one with cold. Avoid extremes of temperature. You want to test temperature discrimination, not how much heat or cold the Pt can withstand.
4. Because pain and temperature receptors somewhat overlap and share a common pathway in the spinal tract of CrN V and in the trigeminothalamic tract, disease generally affects both modalities. For screening purposes, testing one tests both. Test temperature discrimination first. If the Pt discriminates temperature normally, and the history does not suggest neurologic disease, you do not need to prick every Pt with a pin to test pain. No one relishes a pinprick, especially about the face. If you approach (attack) a young child with a pin first, you will have ended the entire sensory examination. Yet even an intelligent 3- to 4-year-old child will play the temperature game with the tuning fork. By testing temperature sensation, you avoid leaving a trail of pinpricks across the skin, oozing blood.
H. Testing pain perception
If the history suggests a sensory disorder, test pain sensation over the face after testing temperature discrimination (see pages 382–383).
I. Differentiation of somatization from loss of facial sensation of an anatomical/physiological origin
1. Draw a line across the head in Fig. 10-5 to show the exact dividing line between the part of the head innervated by CrN V and the cervical dermatomes. Compare it with Fig. 10-2 to see how exact your line is.
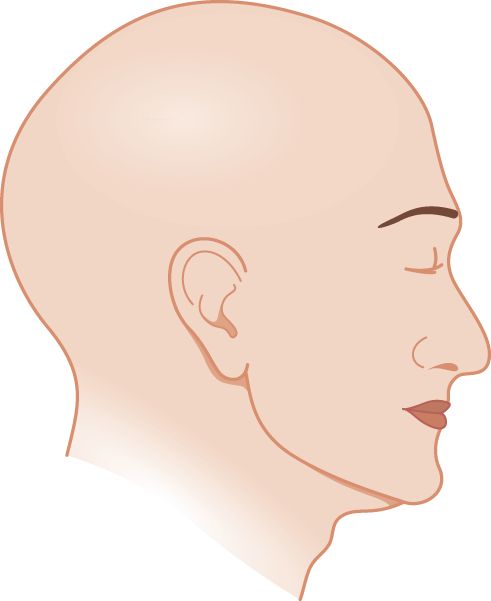
FIGURE 10-5. Blank to draw in the sensory innervation field of the trigeminal nerve.
2. Now shade and label the parts of the face supplied by each of the three major sensory branches of CrN V. Check with Fig. 10-2.
3. Does CrN V innervate the skin over the angle of the mandible? If you included it in your drawing above, check Fig. 10-2 again. You missed a point of importance. Organic sensory loss of facial sensation from trigeminal nerve lesions spares the angle of the mandible, whereas loss of facial sensation from somatic symptom disorder includes it. Review Figs. 2-10 and 10-2 to recall where CrN V abuts on spinal nerve root C2. Comparison of sensory loss on the face from an anatomical/physiological cause versus somatic symptom disorder leads to a useful general law: In Pts with a mental disorder, sensation loss occurs according to their mental image of the body parts; Pts with anatomical/physiological dysfunction lose sensation according to the wiring diagram of the nervous system. As another example, in somatic symptom disorder the loss of sensation in the upper extremity usually cuts off sharply at the wrist, elbow, or shoulder, because that agrees with one’s mental image of an arm, but it does not agree with the actual anatomic distribution of peripheral nerves, nerve roots, or their central pathways (Fig. 14-3).
4. Therefore, in Pts with somatic symptom disorder and sensory loss of facial sensation, the angle of the mandible is  spared/
spared/ affected, whereas in organic loss of affected facial sensation due to a CrN V lesion, the angle of the mandible is
affected, whereas in organic loss of affected facial sensation due to a CrN V lesion, the angle of the mandible is  spared/
spared/ affected. (
affected. ( affected;
affected;  spared)
spared)
J. Analyze this patient
1. This 76-year-old woman complains of severe facial pain, starting 4 months ago and gradually worsening. She describes very brief, shock-like jolts of unbearable pain that runs from the side of her cheek down to the tip of her jaw, on the right side only. She became irascible and impossible to live with according to her husband. Because eating triggers the pain, she has lost 18 lb. The NE discloses normal motor and sensory functions of the CrNs, except that she will not allow the Ex to test sensation over the right lower jaw because touching the right lower lip triggers excruciating shocks of pain (allodynia).
2. In analyzing a sensory complaint, the Ex must determine its nature and whether its distribution conforms to an anatomic pattern or to the person’s mental image of the body and, hence, is psychogenic. The term that best matches the Pt’s sensory disorder is (review the descriptors in Section I B 3a-e before answering):  hyperesthesia/
hyperesthesia/ hyperalgesia/
hyperalgesia/ causalgia/
causalgia/ neuralgia. (
neuralgia. ( neuralgia)
neuralgia)
(Although some other terms more or less apply, neuralgia is the most specific.)
3. Does the location of the sensory disorder match:
 a. The distribution of a central pathway?
a. The distribution of a central pathway?
 b. The distribution of a dermatome?
b. The distribution of a dermatome?
 c. The distribution of a peripheral nerve or nerve branch?
c. The distribution of a peripheral nerve or nerve branch?
 d. A nonanatomic distribution that, in combination with the Pt’s obvious personality change, indicates a psychogenic disorder? (
d. A nonanatomic distribution that, in combination with the Pt’s obvious personality change, indicates a psychogenic disorder? ( c)
c)
4. Which peripheral nerve area does the Pt’s pain correspond to?
_________
5. This Pt had no other abnormalities on the general physical examination or the radiographic examination of the skull with a basilar view to show the foramina of exit of CrN V. She had trigeminal neuralgia (tic douloureux), a very typical neuralgia, characterized by repetitive excruciating shocks of pains in one or more of the branches of the trigeminal nerve. Typically the pain erupts spontaneously and also after touching a “trigger point” on the cheek or the inside of the mouth. It occurs idiopathically with multiple sclerosis and apparently as a result of vascular compression of the root of CrN V by the superior cerebellar artery. Medication or surgical treatment may alleviate it (Reddy and Viswanathan, 2014). There are other causes of facial pain that need to be considered and especially when the clinical presentation is atypical for trigeminal neuralgia (Zakrzewska, 2013).
K. Summary of the tests for the sensory functions of the cranial nerves
Get a partner and rehearse Section V, steps C and D, of the Standard NE.
BIBLIOGRAPHY · Examination of Sensory Functions of Cranial Nerve V
Brodsky H, Dat Vuong K, Thomas M, et al. Glabellar and palmomental reflexes in parkinsonian disorders. Neurology. 2004;63:1096–1098.
Derbyshire SWG. Can fetuses feel pain? BMJ. 2006;332:909–912.
Guberman A. Clinical significance of the corneomandibular reflex. Arch Neurol. 1982;39:578–581.
Hooker D. The Prenatal Origin of Behavior. New York, NY: Hafner Publishing Co; 1969.
Okuda B, Kodama N, Kawabata K, et al. Corneomandibular reflex in ALS. Neurology. 1999; 52:1699–1701.
Okuda B, Kawabata K, Tachibana H, et al. Primitive reflexes distinguish vascular parkinsonism from Parkinson’s disease. Clin Neurol Neurosurg. 2008;110:562–565.
Reddy GD, Viswanathan A. Trigeminal and glossopharyngeal neuralgia. Neurol Clin. 2014;32:539–552.
Siemionow M, Gharb BB, Rampazzo A. The face as a sensory organ. Plast Reconstr Surg. 2011; 127:652–662.
Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–285.
Valls-Sole J. Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin Neurophysio. 2012;123:13–20.
Wilson EK. Ex utero: live human fetal research and the films of Davenport Hooker. Bull Hist Med. 2014;88:132–160.
Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. Brit J Anaesth. 2013;111:95–104.
V. EXAMINATION OF SOMATOSENSORY FUNCTIONS OF THE BODY AND EXTREMITIES
A. Testing light touch sensation
1. Test touch over the rest of the body exactly as described for the face. For screening purposes, test only the dorsum of the hands and feet, in addition to the face. The history determines how far to extend the sensory examination. Review Fig. 2-10 to understand why the Ex would move the touch stimulus up and down the trunk to discover a dermatomal loss or spinal cord sensory level but around the limbs.
2. Quantitative testing of touch and pressure can be done with graded monofilaments or hairs (von Frey hairs) of different strengths or other forms of quantitative sensory testing (Gilron et al, 2015).
3. Review the anatomy of touch in Fig. 2-28.
a. Recall that touch impulses ascend to the somesthetic cortex by two spinal pathways: one in the _________
b. The dorsal column pathway decussates at the _________
4. The pathway for touch in the ventrolateral columns most closely resembles the pathway for  pain and temperature/
pain and temperature/ vibration and position sense. (
vibration and position sense. ( pain and temperature)
pain and temperature)
a. These two tracts, the ventral and lateral spinothalamic tracts, run together as the _________
b. All lemnisci unite in the brainstem to travel to the thalamus as one with the medial lemniscus. Complete Table 10-1.
TABLE 10-1 • Origins and Names of the Lemnisci
Site of origin of axons of lemniscus | Name of lemniscus |
Trigeminal sensory nuclei | |
Cranial nerve VIII nuclei | |
Dorsal column nuclei | |
Dorsal horn nuclei |
(Trigeminal
Lateral
Medial
Spinal)
c. The one sensation that lacks a lemniscus and a specific thalamic relay nucleus is mediated by CrN _________
B. Testing temperature sensation from the body and extremities
Proceed exactly as described for the face. Test temperature discrimination first. Use the tuning fork and finger or warm and cold tube tests (Fig. 10-4).
C. Physiology and anatomy of pain
1. Relation of pain and temperature sensation to types of peripheral axons (Gilron et al, 2015).
a. Prick yourself with a pin. After a pinprick, you will experience two types of pain: fast and slow. Small myelinated fibers of the A group mediate the first pain, a sharp, bright, localized “fast” pain. This pain is mediated mainly through the classic lateral spinothalamic pain and temperature pathway. Small myelinated axons (IA γ fibers) also mediate cold sensation.
b. Unmyelinated C fibers mediate the second type of pain, an afterglow of dull, diffuse, stinging, and burning pain. Diffuse polysynaptic pathways rather than solely the classic spinothalamic pathway likely mediate this pain. This pain connects with the reticular formation and ultimately the limbic system. The small, unmyelinated axons (C fibers) also convey warm sensations. In contrast, the largest fibers of the peripheral nerves mediate the so-called dorsal column modalities (Section V).
2. Clinical classification of pain
a. Pain can be classified as nociceptive and neurogenic (neuropathic) (Video 10-3).

Video 10-3. First bite pain following parotid surgery.
b. Pain of nociceptive origin is divided into somatic and visceral and arises from some local lesion, such as an invasive carcinoma or trauma that stimulates local pain endings.
c. Pain of neurogenic origin arises from some form of heightened sensitivity or over activity from a lesion that affects the peripheral or central nervous system, apart from stimulation of local pain endings. A wide variety of agents, such as a herniated disc, neuropathies, or central lesions, cause neuropathic pain. Neurogenic pain may be mediated through the sympathetic nervous system, nonsympathetically mediated or centrally (Bautista et al, 2014; Denk et al, 2014).
d. The debilitating types of neurogenic pain, that is, neuralgias, such as trigeminal neuralgia and CRPS were described in Section I. In these and similar neurogenic pain syndromes such as erythromelalgia, the sensory examination may be difficult or inconclusive (Dabby, 2012). For such Pts, a simple rating scale provides additional insight into the severity and nature of the pain (Khorsan et al, 2010).
e. Referred pain: The site at which the Pt feels the pain may not correspond to the site of the painful stimulus (Arendt-Nielsen and Svensson, 2001). Thus, cardiac pain is referred down the left arm. In carpal tunnel syndrome, with median nerve compression at the wrist, the Pt may feel pain proximally in the arm and distally in the distribution of the median nerve. The mechanism of referred pain is not known, but several different hypothesis exist as to why it may occur.
3. Anatomy of the pain and temperature pathways from the body and extremities
a. Learn Fig. 10-6 and review Fig. 2-28.
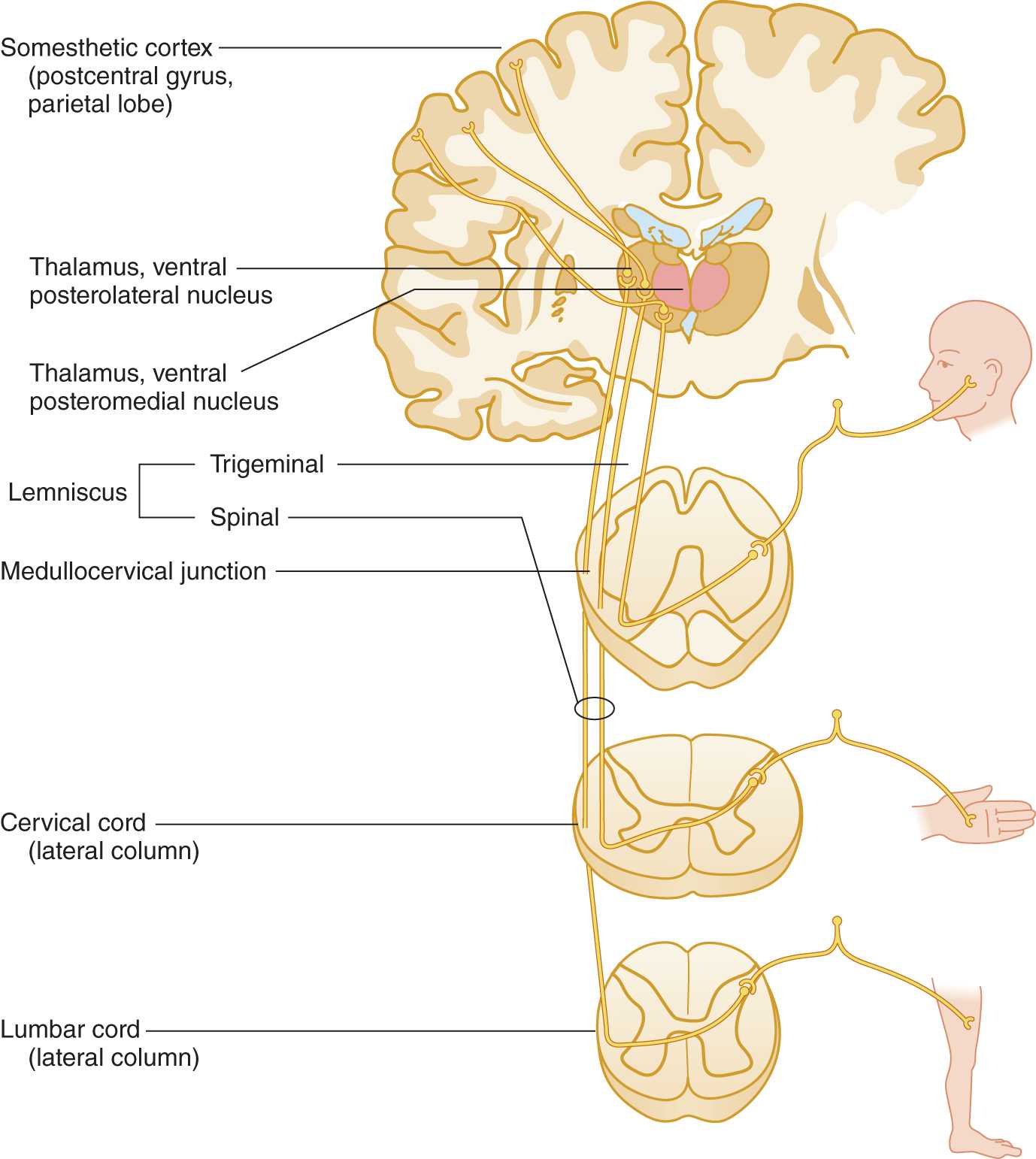
FIGURE 10-6. Pathway for pain and temperature sensation from the periphery to the cerebral cortex. Notice that the sensory axons from the face descend in the descending root of cranial nerve V into the rostral end of the cervical cord.
b. Draw and verbally trace a nerve impulse for pain and temperature sensation from the skin on the lateral side of the foot to the cerebral cortex. Start by enumerating the dermatome.
_________
_________
_________
4. The pain and temperature axons from the foot, trunk, and hand synapse on secondary neurons at, or within one or two segments of, their level of entry into the spinal cord, but the axons from the face descend through the brainstem to reach their secondary neuron. Review Figs. 10-2 and 10-6.
a. The descent of the axons from the face permits the secondary nucleus that relays pain and temperature sensation to be continuous from the medulla to the last sacral segment. The somatotopic pattern in this continuous long columnar nucleus is face, neck, arm, trunk, lower extremity, and sacral region. This nucleus, called the substantia gelatinosa of Rolando, that relays pain and temperature sensations occupies the tip of the dorsal horn.
b. A surgeon who wanted to abolish only pain and temperature sensation without abolishing touch would make a cut in the  ventrolateral/
ventrolateral/ dorsal column to interrupt the _________
dorsal column to interrupt the _________ ventrolateral; lateral spinothalamic)
ventrolateral; lateral spinothalamic)
c. At one time neurosurgeons made such an incision, called a cordotomy, in the ventrolateral column of the spinal cord to relieve Pts of intractable pain. Would this cut eliminate touch sensation?  Yes/
Yes/ No. Explain. (
No. Explain. ( No)
No)
_________
_________
d. In addition to the foregoing classic spinothalamic pathway, a multisynaptic pain pathway ascends from the face, body, and extremities to reach the thalamus and conscious appreciation (Gilron et al, 2015).
e. Positron emission tomography discloses several cerebral areas that mediate the various affective aspects of pain: the anterior and posterior cingulate and inferior frontal cortices, and periventricular gray matter (Tolle et al, 1999; Garcia-Larrer, 2012; Saab, 2012).
D. Testing for pain sensation
1. Instructions for the Pt: Show the Pt a straight pin with its sharp and dull ends. Ask the Pt to respond sharp or dull when you apply the pin. Then have the Pt shut the eyes to prevent visual cues.
2. Use of the pin: Alternate touching the Pt with the two ends of the pin randomly to monitor the Pt’s attentiveness and reliability. Hold the shaft of the pin lightly between the thumb and the index finger, as if to allow it to slip a little, thus applying the stimulus with the same pressure. Make about three successive pricks for each stimulus because not all individual pricks will hit a pain-sensitive spot. Start with a normal area to establish communication, so that the Pt knows what to expect. Test the face and dorsum of the hands and feet. Avoid the horny skin of the palms and soles. Recall the different sensitivities of various skin areas. Always discard the pin after use. Never use it again. We do not know how many angels can dance on the point of a pin, but several diseases can.
3. Delayed pain and deep pain perception: If the history and examination suggest a sensory disturbance, proceed as follows to test the extremities, but do not use these tests on the face.
a. Testing for delayed pain: Pinch the dorsum of the Pt’s foot briskly between the fingernails of your thumb and index finger. The normal person feels pain almost immediately. A delayed response indicates an abnormality in sensory conduction.
b. Testing for deep pain: Test by squeezing very hard on an Achilles tendon (called Abadie sign when the Pt feels no pain) or a muscle or by compressing very hard over a bony surface. The Pt may feel a delayed pain some seconds after ending the compression. Classic causes of delayed pain or absent deep pain are tabes dorsalis and other diseases that interrupt dorsal roots and dorsal columns.
4. Location of tender points: When examining Pts with any acute or chronic pain syndrome, palpate along nerves, muscles, and bony prominences for pain and trigger points. Compress the sites with the ball of the thumb by using firm pressure but short of causing pain in normal persons. Counting the number of tender points assists in the diagnosis of the fibromyalgia syndromes (Clauw, 2014; Fernández-de-las-Peñas and Dommerbolt, 2014).
5. Pain in neonates and fetuses: By custom physicians have done procedures such as circumcision on newborn infants without the use of anesthetics, as if the infant felt no pain. Certainly newborns and fetuses show the behaviors associated with pain: crying, agitation, and autonomic responses (Walker, 2013). Because infants do react to pain or cold, the Ex can ascertain the sensory level in an infant with a spinal cord transection. Start with the infant quiet. Apply a pin or ice cube to the feet and work up slowly toward the neck. The infant will cry as soon as the stimulus reaches an intact dermatome.
6. Ancillary methods of investigating pain
a. Although available, quantitative or automated methods for testing touch, pain, and temperature are not in routine use (Pavlaković and Petzke, 2010; Gandhi et al, 2011; Bakkers et al, 2013). For compression tests to elicit reactions in comatose Pts, see Chapter 12.
b. Skin biopsies provide an additional method of investigating the integrity of small, unmyelinated nerve fibers (Tavee et al, 2014). Sural nerve biopsy likewise aids in categorizing neuropathies (Herrmann et al, 1999; Mikella et al, 2013; Ton and Kruize, 2013).
7. Some have remarked that the function of the internist or the surgical consultant is to do the rectal examination (and discover a prostatic carcinoma that has caused the Pt’s baffling weight loss and back pain). Physicians habitually neglect the same things, such as the rectal examination, and pain and temperature testing. As a neurologist, my function sometimes is to do the temperature and pain testing that no one else has done, and frequently it pays off.
BIBLIOGRAPHY · Examination of Somatosensory Functions of the Body and Extremities
Arendt-Nielsen L, Svensson P. Referred muscle pain: basic and clinical findings. Clin Pain. 2001;17:11–19.
Bakkers M, Faber CG, Peters MJH, et al. Temperature threshold testing: a systematic review. J Peripher Nerv Syst. 2013;18:7–18.
Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17:175–182.
Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547–1555.
Fernández-de-las-Peñas C, Dommerholt J. Myofascial trigger points: peripheral or central phenomenon? Curr Rheumatol Rep. 2014;16:395.
Dabby R. Pain disorders and erythromelalgia caused by voltage–gated sodium channel mutations. Curr Neurol Neurosci Rev. 2012;12:76–83.
Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosc. 2014;17:192–200.
Gandhi M, Sesek R, Tuckett R, Morris-Bamberg SJ. Progress in vibrotactile threshold evaluation techniques: a review. J Hand Ther. 2011;24:240–256.
Garcia-Larrer L. Insights gained into pain processing from patients with focal brain lesions. Neurosci Lett. 2012;520:188–191.
Gilron I, Barin R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90:532–545.
Herrmann DN, Griffin JW, Hauer P. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640.
Khorsan R, York A, Coulter ID, et al. Patient-based outcome assessment instruments in acupuncture research. J Altern Complement Med. 2010;16:27–35.
Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35:373–381.
McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82:737–755.
Mikella CB, Chana AK, Steina GE, et al. Muscle and nerve biopsies: techniques for the neurologist and neurosurgeon. Clin Neurol Neurosurg. 2013;115:1206–1214.
Pavlaković G, Petzke F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr Rheumatol Rep. 2010;12:455–461.
Saab CY. Pain-related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci. 2012;34:629–637.
Tavee JO, Polston D, Zhou L, et al. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve. 2014;49:564–569.
Tolle TR, Kaugman T, Siessmeier T, et al. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol. 1999;45:40–47.
Ton E, Kruize AA. When and how to perform biopsies in a patient with a (suspected) connective tissue disease. Best Pract Res Clin Rheumatol. 2013;27:209–236.
Walker SM. Biological and neurodevelopmental implications of neonatal pain. Clin Perinatal. 2013;40:471–491.
VI. EXAMINATION TECHNIQUES IN PERIPHERAL NEUROPATHIES
A. Classification of neuropathies
1. Sensory or sensorimotor neuropathies fall into two main types: diffuse symmetrical polyneuropathies and focal neuropathies or mononeuropathies. A second major classification consists of pure sensory, pure motor, and combined sensorimotor neuropathies. The disease may cause an autonomic neuropathy, somatic neuropathy, or combined autonomic-somatic neuropathy (Alport and Sander, 2012; Barohn and Amato, 2013).
2. Diffuse symmetrical polyneuropathies generally cause sensory disturbances in the distal part of the upper and lower extremities. The Pt has a “stocking and glove” distribution of numbness, tingling, paresthesias, dysesthesias, and pain (Fig. 10-7).
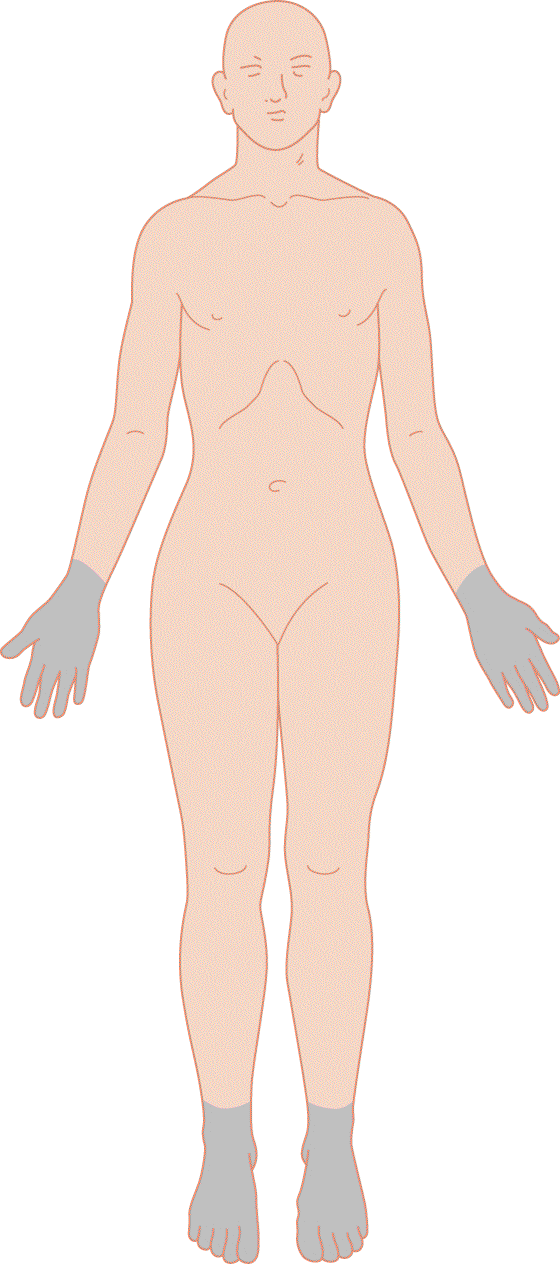
FIGURE 10-7. Areas of sensory loss caused by a diffuse, symmetrical polyneuropathy. The demarcation between affected and unaffected areas fades gradually. Sensory loss in somatic symptom disorder tends to cause sharp margins between the affected and unaffected zones. Compare with Fig. 14-7.
a. The sensory examination is straightforward. The Ex compares the results of testing pain, temperature, and light touch distally in the extremities with the results of testing proximally. Common causes of polyneuropathies are toxins, drugs, alcoholism, autoimmune disorders, and heredity (London and Albers, 2007).
b. Some symmetrical polyneuropathies cause palpable enlargement of nerve trunks. Inspect and palpate the nerves that cross the posterior triangle of the neck, the ulnar, and the common peroneal nerves. Leprosy also may enlarge peripheral nerves.
c. Mononeuritis multiplex, such as in diabetes mellitus or a vasculitis, may imitate diffuse symmetric neuropathies.
3. Focal neuropathies or mononeuropathies generally result from entrapment or mechanical compression, but metabolic disorders may predispose to them and may coexist (Arnold and Elsheikh, 2013). The entrapment neuropathies often cause extreme pain. The investigation involves a search for trigger factors, often a posture or position, that will elicit or exacerbate the symptoms and for any postures or positions that relieve the discomfort. Compression or percussion over the site of entrapment often will trigger or reproduce the sensory symptoms distal to the entrapment site, a phenomenon called Tinel sign (dysesthesias produced by tapping over the nerve). The phenomenon is equivalent to the pain that radiates down into your little finger when you bang your ulnar nerve at the elbow, called “hitting your crazy bone.” Local anesthetic block at the entrapment site abolishes the sensory disturbance and Tinel sign in the entrapment neuropathies. The text will describe special features of the NE for some of the more common entrapment neuropathies. Granted the importance of corroborative findings on the examination, the diagnosis depends very strongly on the history. Some Pts with entrapment neuropathies by history will not show changes by the clinical or electrophysiologic examination. Most of the entrapment neuropathies respond well to surgical decompression, if conservative therapy fails.
4. Ancillary tests include quantitative sensory testing, electromyography (EMG) and nerve conduction tests, metabolic screening tests, biopsy of sensory nerves or nerve endings in skin, cerebrospinal fluid examination, genetic testing, and sometimes magnetic resonance imaging (MRI) (Andreisek et al, 2006; Whittaker, 2012; Bromberg, 2013; Saporta 2014; Staff and Windebank, 2014). The Ex can follow the course of neuropathies over long periods by combining clinical and laboratory results into a total neuropathy score (Griffith et al, 2010).
B. Special features in the examination for entrapment neuropathies
1. Occipital neuralgia causes pain radiating from the base of the skull up the occiput. The Pt may notice positions of the head that elicit the pain. Palpate the occipital region for masses, such as lymph nodes. Tap (Tinel sign) and use the ball of your thumb to compress over the site where the greater occipital nerve exits from the fascial ring, about 2 cm caudolateral to the inion. Local anesthetic block may be tried in doubtful cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


