and Robert E. Schmidt2
(1)
Sunnybrook and St Michael’s Hospitals, University of Toronto, Toronto, ON, Canada
(2)
Division of Neuropathology Department of Pathology, Washington University School of Medicine, St. Louis, MO, USA
This chapter provides a brief sketch of methodology for the preparation of peripheral nerve tissue, followed by a systematic approach to histological examination of a peripheral nerve biopsy, with an emphasis on differential diagnosis.
7.1 Methods
Specimen preparation is fundamental, and four methods should be available for the study of peripheral nerve pathology. Immediately upon excision of the sural nerve, the technologist receives the specimen on a piece of dental wax and removes a 5 mm segment for freezing. The rest of the specimen is placed straightened and slightly stretched on a wooden stick and immediately immersed in cooled 2.5 % glutaraldehyde in 0.025 M cacodylate buffer at pH 7.4 and an osmolarity between 300 and 330 mOsm. After 5 min, portions of tissue are taken for paraffin embedding and fiber teasing; then the specimen is returned to fresh glutaraldehyde.
7.1.1 Paraffin-Embedded Sections
The Saint Michael’s Hospital lab obtains better results with B5 as the main fixative. After about 3 h, the tissue is post fixed in formalin, and segments are selected for cross and longitudinal sectioning. Routine stains include H&E and Congo red (amyloid). Other commonly used stains are Verhoeff–Van Gieson (elastin), Perl’s Prussian blue (iron), fite (acid-fast bacilli, AFB), Martius Scarlet blue, and phosphotungstic acid hematoxylin (PTAH) (fibrin).
Most immunohistochemical reactions are carried out on paraffin-embedded tissue. Commercially available immunohistochemical stains are frequently used including antibodies to CD-3 (T cells), CD-20 and CD79a (B cells), CD68 and CD163 (macrophages), neurofilaments, Ki67 (proliferating cells), CD34 and smooth muscle actin (endothelial cells, vascular wall integrity), collagen IV (cells with basement membranes), epithelial membrane antigen (perineurial cells), and others.
7.1.2 Plastic-Embedded Sections
In the assessment of a nerve specimen, examination of toluidine blue-stained plastic-embedded sections is critical. After 2 h in glutaraldehyde, the nerve is examined under a dissecting microscope. Using a new razor blade and biological forceps, the specimen is subdivided into 3–4 mm long segments; four or five of these are retained intact as large blocks, representing the entire cross-sectional area of the nerve. The rest of the segments of nerve are sectioned in the longitudinal plane which optimally results in 20–30 blocks, each having two to three fascicles. The shape of the blocks, namely, slabs having a recognizable fascicular arrangement, enables technicians to align the nerve bundles properly for transverse and longitudinal sectioning. We believe that tissue from a nerve biopsy should not be diced or cut indiscriminately into small cubes, for such blocks cannot be oriented by the technologist. Penetration of solutions is consistently adequate even for large blocks. This method allows for sections of a useful size for light microscopy and permits examination of the entire cross section of the nerve at multiple levels. A balance must be struck between trimming away sufficient epineurial tissue to facilitate dehydration and infiltration of resin and preserving enough epineurial elements for ultrastructural examination. All blocks are again immersed in new glutaraldehyde overnight or over the weekend. Then, the tissue is post fixed in 1 % OsO4 in Millonig’s buffer at pH 7.4 for 3–5 h at room temperature, followed by dehydration, infiltration with resin, and embedding in Epon-Araldite (Hayat 1989). We have used Embed medium effectively since some constituents used for Epon-Araldite plastic embedding are no longer available. When necessary the processing can be interrupted in the dehydration stage; the tissue can be kept in 70 % alcohol overnight at 4 °C without deleterious effects.
From each biopsy, semithin (approximately 1 μm thick) sections (optimally) from 20 blocks (including all large transversely oriented specimens) are obtained, mounted on glass slides, and stained with toluidine blue. We perform ultrastructural studies of thin sections stained with uranyl acetate and lead citrate from selected blocks in nearly one-half of cases, including those containing all fascicles. The use of a diamond knife facilitates sectioning of large blocks, but similar results can be obtained with standard glass knives. Mounting of sections on wide-mesh grids or formvar-coated slot grids permits a better appreciation of endoneurium on low-power EM examination.
7.1.3 Frozen Sections
Tissue frozen at the time of biopsy may be used for biochemical studies, stained for lipids, and used in immunofluorescent studies.
7.1.4 Fiber Teasing
Although fiber teasing is not routinely performed at our institutions, a 1–1.2 cm nerve segment taken immediately after biopsy is fixed in formalin, post fixed in osmium tetroxide in Millonig’s buffer at pH 7.4 for 3–5 h at room temperature, and passed through graded glycerin from 30 to 100 %, where it may be kept for several weeks before dissection.
7.2 Approach to Specimen Examination
7.2.1 Biopsy Examination
Biopsy examination includes:
1.
Assessment of the fascicular anatomy and specimen quality
2.
Light microscopy
Epineurium
Perineurium
Endoneurium
Neuropathic process
Other pathology
3.
Ultrastructural examination (selected tissue blocks)
4.
Immunohistochemical examination (specific circumstances)
5.
Fiber teasing, morphometry (rarely used)
7.2.2 Essential Points to Be Addressed by the Examination
Essential points to be addressed by the examination are:
1.
Is there evidence of peripheral neuropathy?
2.
Is the pathological process axonal, demyelinating, or both?
3.
Is the pathological process acute or chronic?
4.
Are there any features permitting a specific etiologic diagnosis?
In over half of the cases, no specific features are found. In such instances, we provide a differential diagnosis and our impression of the most likely etiology. We make every effort to secure clinical, laboratory, and electrophysiological information with each nerve biopsy. This makes tissue interpretation more meaningful and will sometimes prompt special procedures. For example, if vasculitis, amyloid, or leprosy is strongly suspected and only nonspecific findings are seen on the initial tissue examination, the entire specimen may be sectioned and examined using special stains looking for a diagnostic lesion. Conversely, our observations regarding striking discrepancies between clinical and histological findings may prompt the clinician to reassess the patient, and further enquiry may reveal important information such as a positive family history or a toxic exposure. When indicated (e.g., suspected vasculitis), one can examine frozen sections following biopsy for a rapid diagnosis.
7.3 Assessment of Specimen Quality
Peripheral nerve is highly susceptible to artifacts, which should not be misinterpreted as pathological changes (Table 7.1). Nerve fascicles are round in vivo, a consequence of the elastic properties of perineurium and the pressure gradient between the endoneurial and epineurial compartments. Any deviation from this is likely to be an artifact, although a severely diseased fascicle may also become distorted. Similarly, in vivo myelinated internodes are thought to be round in cross section, excepting the normal crenated appearance at the paranodal area. Another clue to the presence of artifact is the observation of intrafascicular geographic regions of abnormal structure, without evidence of cellular reactive change.
Table 7.1
Artifacts in nerve biopsy specimens (paraffin and resin histology)
Procedure | Appearance | Cause |
|---|---|---|
Biopsy | Acute inflammatory cells in vessels (Fig. 7.9) | Excessively long procedure |
Hemorrhage | Trauma | |
Split of myelin sheath at SL clefts (Fig. 7.1) | Stretch trauma | |
Focal distortion and ballooning of myelin and axon | Stretch trauma | |
Invagination or rupture of vessels or perineurium (Fig. 7.3) | Crush trauma | |
Vacuolation of tissue, loss of stain affinity (Fig. 7.7) | Electrocoagulation | |
Fixation | “Neurokeratin” artifact (Fig. 7.2) | Formalin fixation |
Shrinkage, with crescent-shaped fascicles and loss of fiber circularity (Fig. 7.8) | Hyperosmolarity of primary fixative | |
Swelling of cells, loss of resolution, dissolution of membranes | Delayed fixation, autopsy material | |
Trimming | Oblique myelin profiles | Malorientation |
Perineurial wrinkles | Incomplete trimming of epineurium | |
Incomplete heat stabilization of sections on hot plate | ||
Embedding | “Popping” of sections, usually at sites of lipid accumulation or degradation, when examined under high-intensity electron beam | Type of resin |
Sectioning | Chatter | Dull knife |
Large wrinkles in tissue | Excessive heat on hot plate | |
Staining | Stain precipitate, contamination | Stain not filtered |
Blotchy staining | Vaporization of solution due to excessive heat on hot plate | |
Dark staining with loss of contrast | Sections too thick, excessive staining time |
Partial or full circumferential splits in the myelin sheath are commonly seen (Fig. 7.1a–d). These correspond to Schmidt–Lanterman (SL) clefts, which are extremely fragile structures, enlarged and distorted by the trauma of biopsy. In normal large myelinated fibers, SL clefts can be seen in up to 33–50 % of cross sections, while in small myelinated fibers, they are present in 5–10 %. This difference explains why the myelin splits are so much more common in large myelinated fibers. “Neurokeratin” artifact refers to the herringbone appearance of myelinated fibers on formalin-fixed longitudinal sections or wagon wheel appearance in cross sections (Fig. 7.2a, b). Asbury and Johnson (1978) suggest that this is a result of autolytic myelin vacuolation. Fixation for several hours with B5 prior to formalin markedly reduces the occurrence of this alteration, and it is not seen in glutaraldehyde-fixed material.
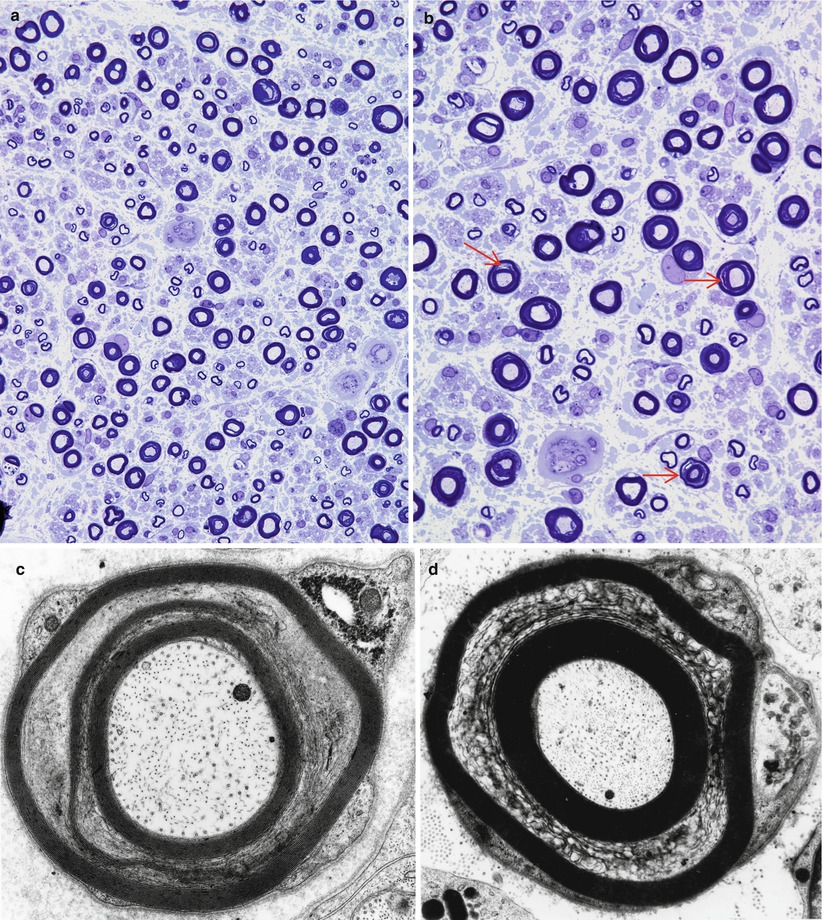
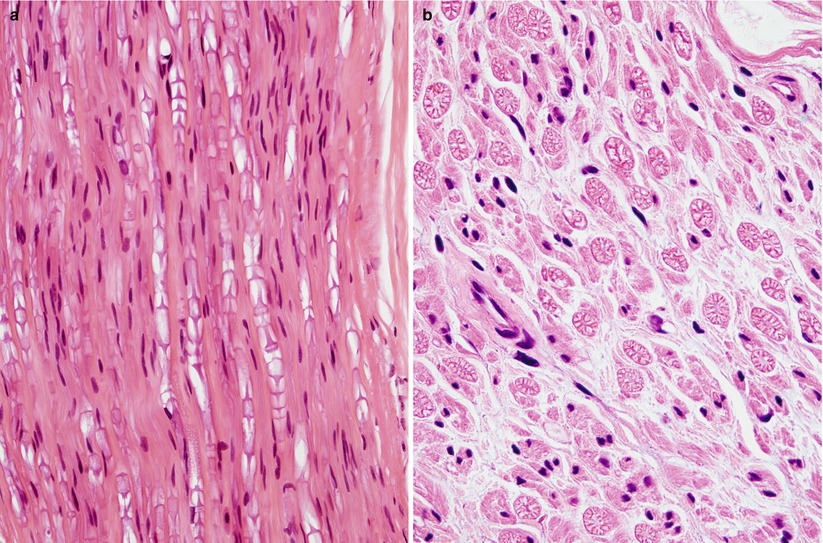

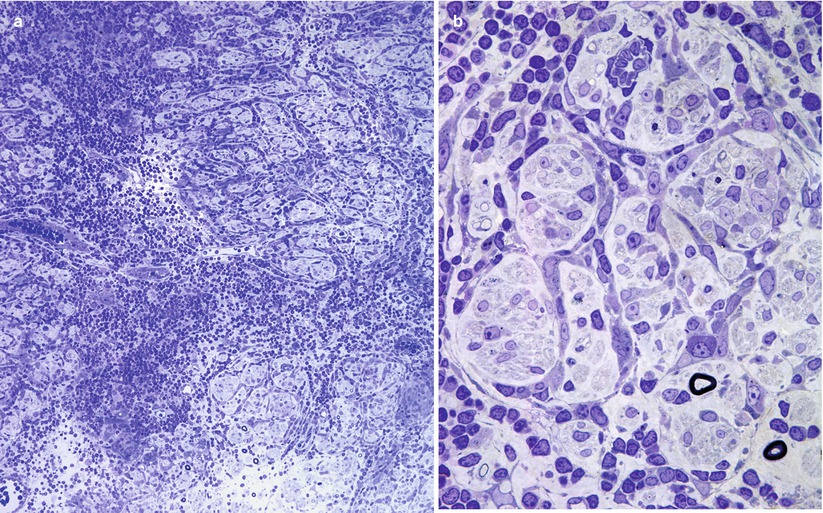
Fig. 7.1
Stretch artifact. Myelin splits (arrows, b) show a vague resemblance to uncompacted myelin. Large MFs bear the brunt of the insult (a, b 1 μ thick toluidine blue-stained plastic sections, a 400×; b 1,000×; c, d electron micrographs, c 21,600×; d 11,440×)

Fig. 7.2
Neurokeratin artifact shown in longitudinal (“herringbone,” a) and cross section (“wagon wheel,” b) is commonly seen after formalin fixation (paraffin, 600×)
The nerve biopsy procedure has been described in detail (Dyck et al. 1993; Asbury and Johnson 1978). Meticulous technique and delicate handling are essential. Electrocoagulation should not be used during any part of the procedure. While the pathologist is always requesting longer sections of nerve, the surgeon naturally wishes to minimize the size of the incision and we continually are challenged by short segments of nerve with significant superimposed artifact. One unfortunate approach is to pull the nerve down through a small incision, cut a long section of nerve, and then allow the cut end of the nerve to retract deeply into the tissue layers! While this is reasonable for harvesting nerve graft material, it is unacceptable for diagnostic nerve biopsy.
Recognition of artifact is an important part of interpreting nerve biopsies, particularly when contributed by outside institution without our control of fixation and handling. Distorted nerve fascicles and crushed vessels are typically seen near the ends of biopsy specimen (Figs. 7.3a–d); the vessels telescope internally and may superficially appear to be scarred (Fig. 7.3c). Myelin can sometimes disintegrate around an apparently intact axon through splitting at the major dense line, creating a net-like pattern of vesicles 80 nm in diameter. This alteration is seen both as artifact and as part of a variety of neuropathies. The case shown (Fig. 7.4a–c) represents delayed fixation in an autopsied patient and, thus, most assuredly artifact. Delayed fixation may also cause swelling and clearing of the cytoplasm of Schwann cells (Fig. 7.4d). An unusual artifact is creation of “two-toned myelin” (Fig. 7.5a–c) in which there is uneven osmication of the myelin sheaths, likely a result of problems with osmium penetration (King 1999). A common alteration is shown in Fig. 7.6a, b resulting in focal apparent loss of myelin structure which is thought to represent problems with dehydration of the specimen during processing. Thermocoagulation of the nerve produces a vacuolated appearance (arrow, Fig. 7.7a) and makes interpretation of pathology almost impossible and mimics the appearance of active axonal degeneration (Fig. 7.7b).
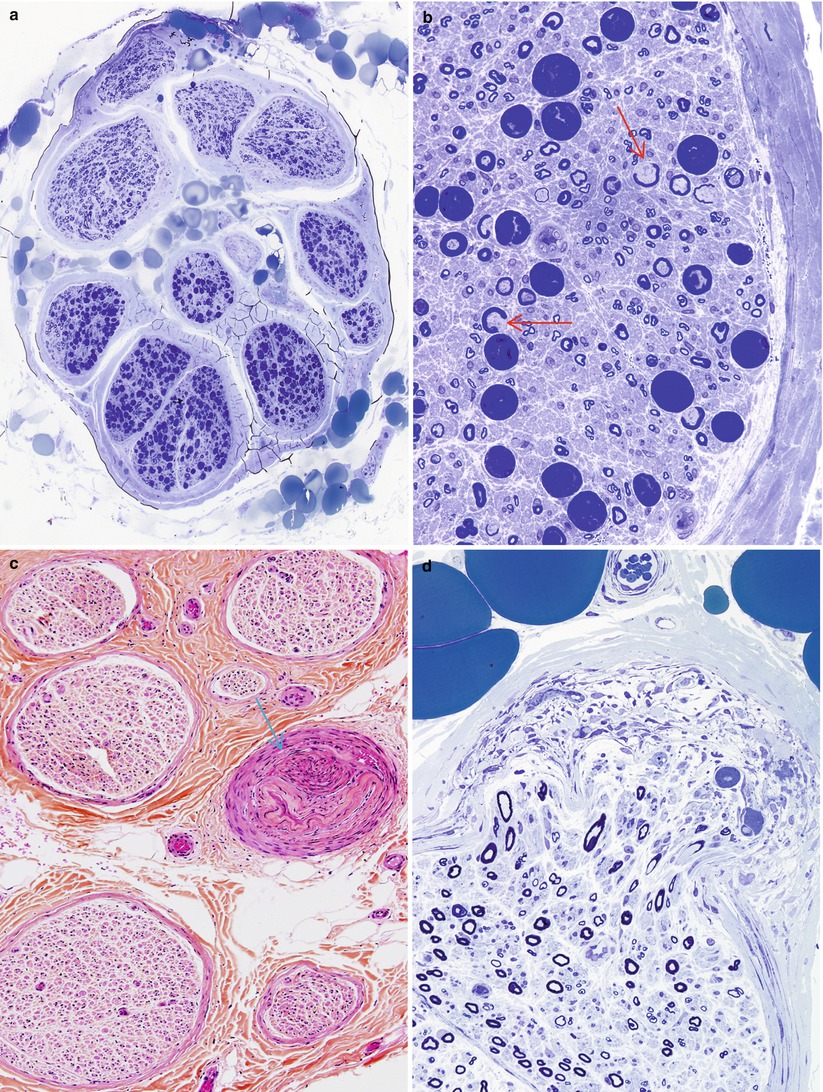
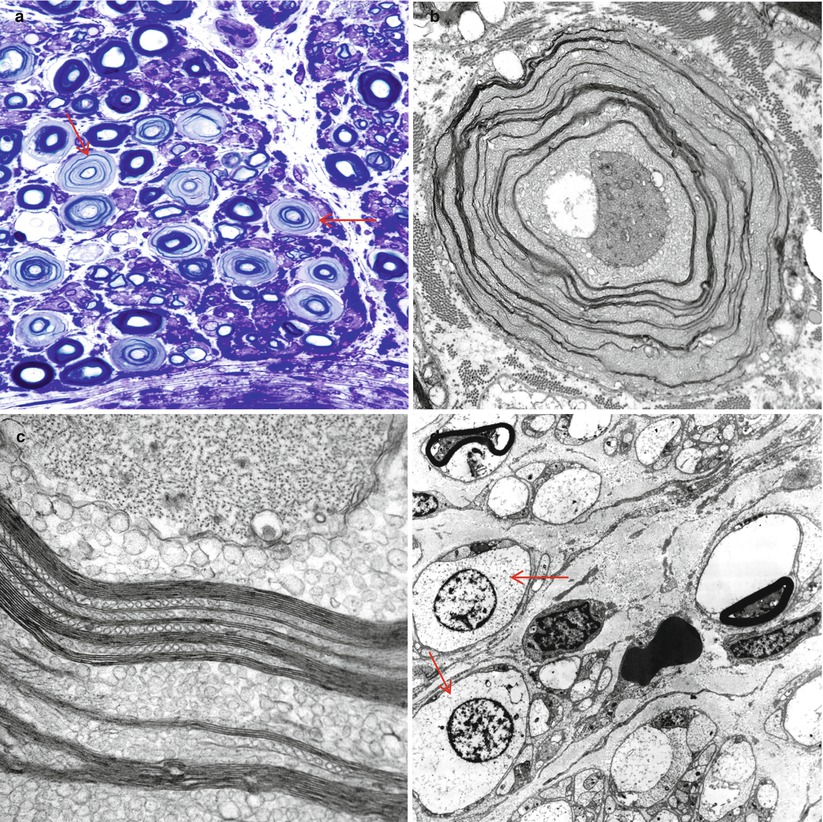
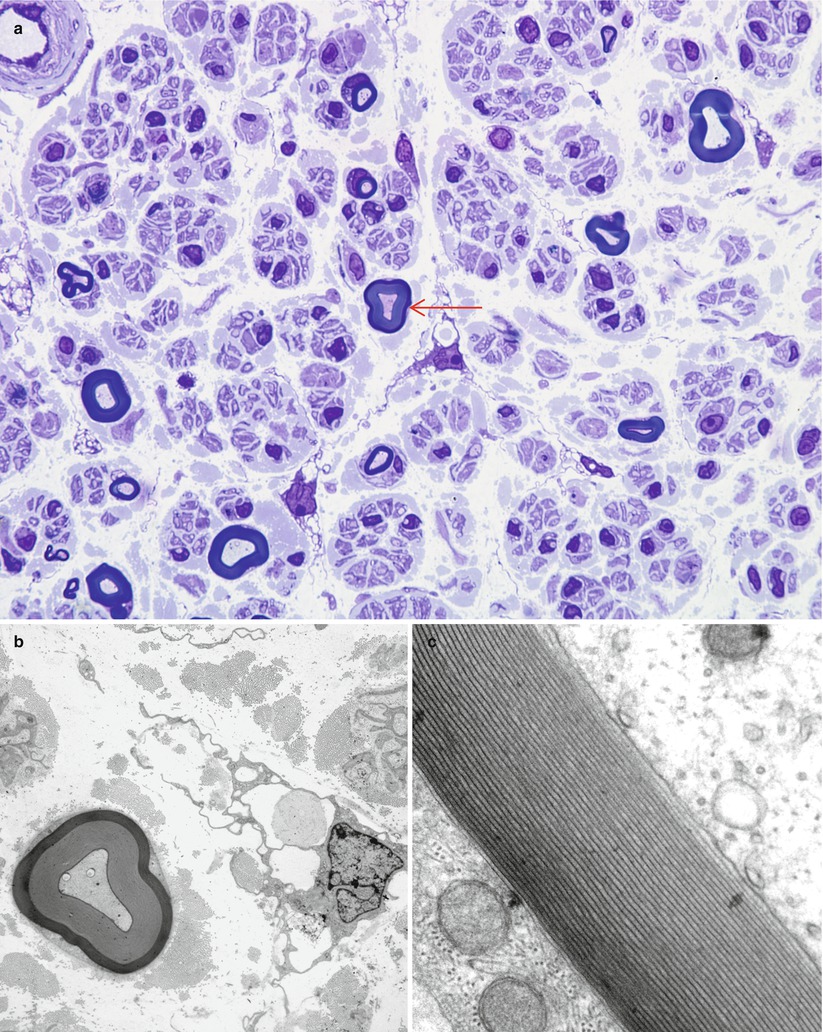
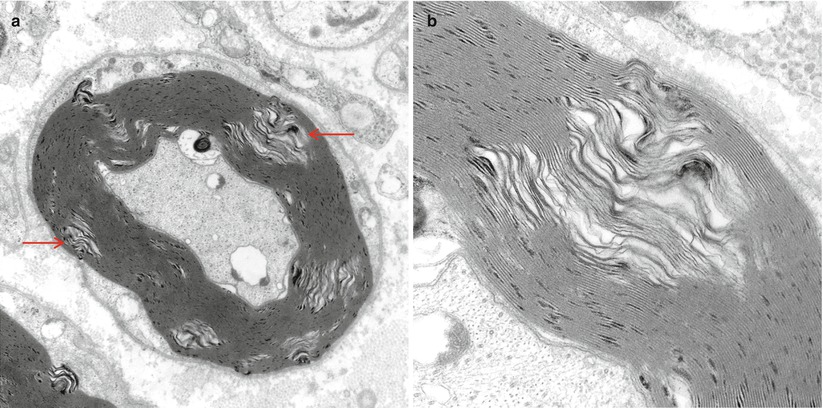
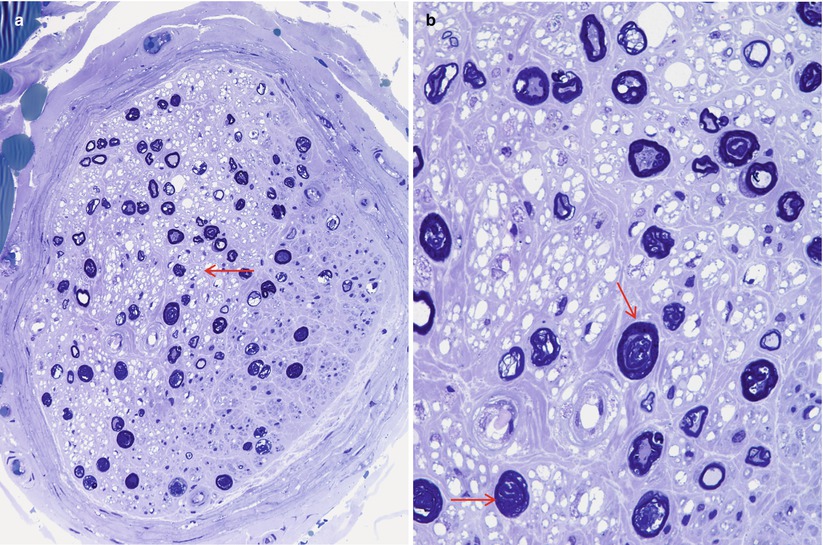

Fig. 7.3
Crush artifact. (a, b) Note collections of dark staining myelin involving the inferior fascicles of nerve (a) which consists of artifactual swellings of MFs with extrusion of axoplasm through artifactual gaps in the myelin sheath (arrows, b). (c) An artifactually telescoped epineurial arteriole (arrow) may be misinterpreted as healed vasculitis. In (d) disruption of the perineurium with extrusion of endoneurial contents is seen (a, b 1 μ thick toluidine blue-stained plastic sections, 400×; c hematoxylin phloxine saffron, HPS trichrome, 200×; d, 1 μ thick toluidine blue-stained plastic sections, 600×)

Fig. 7.4
Delayed fixation artifact. (a) toluidine blue stains show replacement of normal myelinated axon appearance by pale expanded sheaths (arrows). (b, c) Ultrastructural examination shows that pale sheaths represent myelin which has been completely replaced by vesicular myelin (b), seen at higher magnification in (c). (d) Marked swelling and vacuolation of Schwann cell cytoplasm (arrows) in autopsied patient (a 1 μ plastic section, 1,000×; b 12,000×; c 50,000×; d 3,000×)

Fig. 7.5
Uneven myelin staining (“two-tone myelin”). Altered density of the lamellae thought to reflect uneven osmication (arrow, a). (b, c) Ultrastructural examination of the same axon shown in (a) mimics changes in myelin periodicity, a result which is not confirmed at higher magnification (c) (a 1 μ toluidine blue-stained plastic section, 1,000×; b electron micrograph, 5,000×; c 100,000×)

Fig. 7.6
Intramyelinic gaps. These pale separations of myelin seen at low (arrows, a) and high magnification (b) are thought to represent dehydration artifact during processing (King 1999) (a 15,000×; b 50,000×)

Fig. 7.7
Electrocoagulation artifact. Note vacuolation and pallor of endoneurium (arrow, a). At higher magnification, the distortion and ballooning of large MFs (arrows, b) mimics Wallerian degeneration (1 μ thick toluidine blue-stained plastic sections) (a 400×; b 1,000×)
The importance of optimal fixation for accurate morphometry has been repeatedly demonstrated. The endoneurial area of fixed nerve is reduced by 10–43 % relative to snap-frozen nerve (Behse et al. 1974; Dyck et al. 1979). The most important determinant of the degree of shrinkage is the osmolarity of the fixative solution, with a 10 % shrinkage in iso-osmolar fixative (~300 mOsm) and 43 % shrinkage in hyperosmolar fixative (~640 mOsm) (Fig. 7.8a, b). Whether the increased osmolarity results from higher glutaraldehyde concentration or the addition of sucrose is of no consequence (Dyck et al. 1979). Separation between myelin lamellae is decreased by about 17 % with iso-osmolar fixation (Behse et al. 1974). Axonal area and circularity similarly decrease with increasing fixative osmolarity; a difference of 10–18 % was seen with osmolarity of 900 mOsm compared to 410 mOsm, depending on axon diameter (Ohnishi et al. 1976; Holland 1982). Increasing duration of glutaraldehyde fixation is claimed to have a similar impact: axonal area was reduced by 17 % when fixation for 1 and 12 h was compared (Ohnishi et al. 1974). This was not accompanied by a reduction in fascicular area, and the extra space was said to fill by clefts in the endoneurium (Ohnishi et al. 1974). However, we have noticed only minimal changes in the nerve specimen even when nerves are left in glutaraldehyde over a weekend.
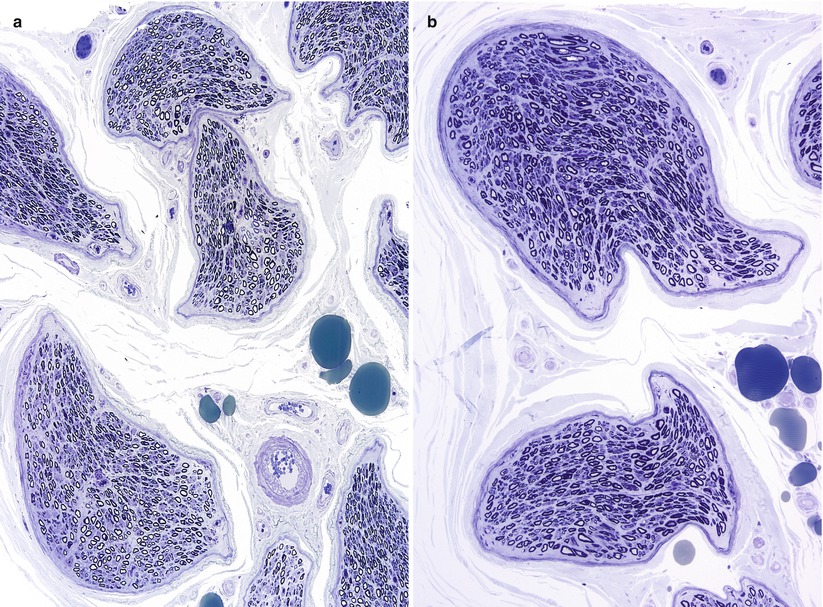

Fig. 7.8
Hyperosmolar artifact. (a, b) Note shrunken crescentic fascicles and loss of fiber circularity (1 μ thick toluidine blue-stained plastic sections [a 100×; b 400×])
7.4 Light Microscopy
7.4.1 Examination of the Epineurium
7.4.1.1 Fascicular Anatomy
It is worthwhile to record the number and shape of fascicles and quantity of epineurium provided by the biopsy. The typical sural nerve contains 6–15 fascicles, but we have received specimens with only two or three fascicles, perhaps representing an accessory peroneal nerve or the non-fused posterior tibial or peroneal components of the sural nerve. The sensitivity of the biopsy for detection of vasculitis, amyloidosis, malignant cell infiltrations, or granulomata is proportional to the amount of tissue available for examination.
Visibly enlarged nerves suggest a number of diagnoses (Table 7.2). Obliteration of the epineurial and fascicular architecture can be seen in tuberculoid or borderline leprosy.
Table 7.2
Diseases causing nerve enlargement
Leprosy |
“Onion-bulb” neuropathies |
CMT-1, Dejerine–Sottas (CMT-3) |
CIDP |
Refsum disease |
Interstitial enlargement |
Amyloidosis |
Acromegaly |
Nerve neoplasm |
7.4.1.2 Epineurial Vessels
Arterioles, venules, variable amounts of adipose tissue, and occasional elastic fibers are recognized in the epineurium. Adipose cells appear as optically empty spaces on paraffin sections, whereas in plastic sections, they exhibit varying degrees of osmiophilia; osmium tetroxide fixation prevents dissolution of fat in the alcohol bath. Toluidine blue staining of semithin sections results in green staining of fat when it has not been extracted during processing. Blood vessels in the epineurium are predominantly oriented longitudinally, although some transverse and oblique profiles can be detected at points of branching. Assessment of vessels should begin on paraffin sections with a search for evidence of acute or remote vasculitis (Fig. 7.10, Table 13.2). The caliber, location, and type of vessels most involved should be assessed: epineurial, perineurial, or endoneurial or arterioles or venules. One must avoid overcalling nondiagnostic vascular alterations (Fig. 7.11a, b), even in a patient with appropriate history. When an active inflammatory infiltrate is detected, the cell types should be identified (Table 7.3).
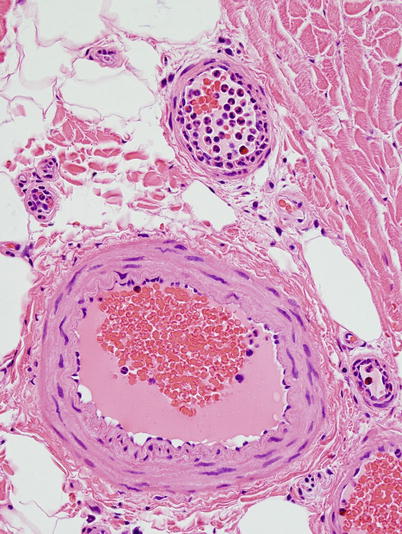
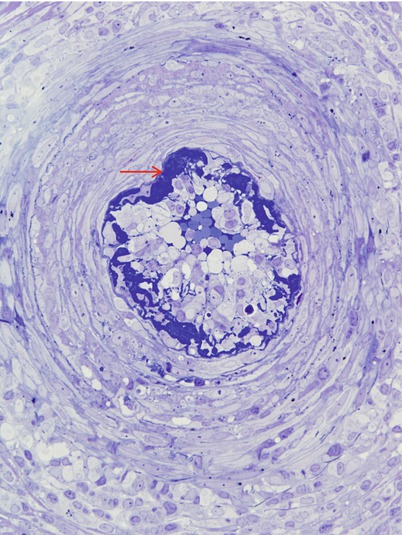

Fig. 7.9
Neutrophil margination and migration through vessel wall is seen in prolonged surgical procedures (paraffin, H&E, 400 ×)

Fig. 7.10
Acute necrotizing vasculitis: In semi-thin sections fibrinoid necrosis is osmiophilic and dark blue (arrow). (1 μ thick toluidine blue stained plastic sections)
Table 7.3
Significance of neural infiltrates
Cell type | Significance |
|---|---|
Lymphocytes | Suggest vasculitis if within vessel wall but otherwise nonspecific (see text) |
Neutrophils | Almost always suggest necrotizing vasculitis; however, may represent artifact of excessively long biopsy procedure |
Eosinophils | May appear in any necrotizing vasculitis, but favor Churg–Strauss angiitis if very prominent; may occur in hypereosinophilic syndrome, toxic oil, and eosinophilia–myalgia syndromes |
Foamy cells | Leprosy, late Wallerian degeneration, lipid storage disease |
Epithelioid histiocytes | Sarcoidosis, leprosy |
Polymorphic inflammatory infiltrate | Lymphomatoid granulomatosis, any necrotizing vasculitis, leprosy |
Plasma cells | Plasma cell dyscrasia, syphilis, vasculitis, Lyme disease, leprosy |
Atypical mononuclear cells (monoclonal immunophenotype) | Infiltrative lymphoproliferative disorders, lymphomatoid granulomatosis |
Perivascular cuffing with mononuclear cells is a common observation and is more readily recognized with leukocyte common antigen (LCA, CD45) immunohistostaining or more specifically using immunomarkers for T (CD3) or B (CD20) cells. Several mononuclear inflammatory cells around the occasional epineurial vessel may be seen in noninflammatory neuropathies and in normal nerves and should not be over-interpreted. A more significant infiltrate is abnormal but nonspecific (Table 7.4). A major problem is deciding where brisk perivascular cuffing ends and vasculitis begins, especially in very small vessels and capillaries. Prominent perivascular cuffing around small vessels has sometimes been called “microvasculitis” and has led to some difficulty in interpretation of the literature. We believe the diagnosis of vasculitis requires evidence of vessel injury; otherwise “perivascular cuffing” is a better description, even if occasional inflammatory cells are found within the vessel wall. Examination of serial sections alternately stained with LCA, Verhoeff–Van Gieson, Martius Scarlet blue, and Perl’s ferrocyanide is helpful in this regard. Diffuse or patchy inflammatory cell accumulation, particularly containing atypical cells, in the nerve should suggest the diagnosis of an infiltrative lymphoid malignancy but can also be seen in lymphomatoid granulomatosis, leprosy, and vasculitic neuropathy.
Table 7.4
Inflammatory neuropathies
Necrotizing vasculitis |
Non-necrotizing inflammatory neuropathy in systemic inflammatory disease (Sjogren’s, SLE, etc.) |
Inflammatory demyelinating neuropathy (GBS, CIDP) |
Paraneoplastic neuropathies |
Sensorimotor neuropathy |
Vasculitic neuropathy |
Subacute sensory neuropathy |
Paraproteinemic neuropathy |
Perineuritis |
Neuropathy with graft vs. host disease |
Sarcoidosis |
Infectious diseases |
Leprosy |
HIV DSPN |
Lyme disease |
CMV neuritis |
Chagas disease (Chimelli and Schieber 1994) |
Non-Lyme neuritis associated with insect sting |
Toxic exposure |
Adulterated rapeseed oil intoxication |
Eosinophilia |
Epineurial vascular proliferation provides indirect evidence of vascular compromise and is seen most commonly in remote vasculitis and diabetes but has also been described in Castleman disease and leprosy (Schroder 1986; Donaghy et al. 1989; Jacobs et al. 1993). Very rarely, the intravascular contents themselves can provide a specific diagnosis, such as intravascular lymphoma (Vital et al. 1989) or cholesterol embolus syndrome (Bendixen et al. 1992).
7.4.1.3 Other Elements of the Epineurium
We routinely examine a Congo red-stained cross section of the full nerve under polarized light and/or thioflavin-S by immunofluorescence. We will review multiple sections through the entire block if amyloid is specifically considered by the clinician. Amyloid deposits may be subtle, and in one instance, we observed them only in association with the epineurial adipose tissue. Proliferation of epineurial connective tissue is nonspecific but if particularly massive, may be suggestive of neuropathy associated with scleroderma (Richter 1954). Focal conglomerates of epithelioid cells or frank granulomata suggest the diagnosis of sarcoidosis, but by definition, there should be evidence of a multisystem process (Chap. 10), and additional diagnoses should also be considered (Table 7.5). The rare observation of Pacinian corpuscles and other sensory nerve organs in the epineurium and perineurium is a normal finding that should not be given pathological significance (Fig. 2.3).
Table 7.5
Granulomatous neuropathies
Sarcoidosis |
Noncaseating granulomatous neuropathy (no evidence of systemic disease) |
Leprosy (TT, BT, BB) |
Churg–Strauss angiitis |
Wegener granulomatosis |
Lymphomatoid granulomatosis |
Angioimmunoblastic lymphadenopathy |
7.4.2 Examination of the Perineurium
Some diseases affecting peripheral nerves have a disproportionate impact on the perineurium, usually in the form of perineurial inflammation. The epineurial and endoneurial compartments may be entirely spared, but more often, there is mild but definite inflammation in these regions also, typically around vessels. This is a nonspecific picture, but suggestive of certain diagnostic possibilities (Table 7.6). Leprosy in particular is notorious for its perineurial involvement, and a search for the organism should be conducted with step sections through the specimen using special stains (fite or auramine rhodamine).
Table 7.6
Relatively selective perineurial inflammation
Leprosy |
Idiopathic perineuritis |
Toxic oil syndrome, eosinophilia–myalgia syndrome |
Cryoglobulinemia (unusual) |
Sarcoidosis (unusual) |
Lyme disease (unusual) |
Lymphoma (unusual) |
Calcification of the perineurium is a nonspecific finding in chronic neuropathy (Figs. 2.4b and 2.6b). It is often not detected because tissue preparation methods result in its removal (Van Lis et al. 1979). We have seen perineurial calcification in all three cases of amiodarone neuropathy examined in our laboratory, and this alteration was also significant in a patient with renal failure (Paetau and Haltia 1985). The calcium deposits often have a target-like configuration and are found interspersed between perineurial lamellae. “Ghost bodies” are circular, well-circumscribed, interstitial structures which demonstrate irregular electron density; their presence has been described in a variety of tissues undergoing calcification (Anzil and Palmucci 1983).
Following some nerve injuries, focal proliferation and endoneurial invasion of perineurial cells causes a “microfasciculation.” This is a nonspecific response of perineurial cells to injury and has been described in leprosy (Fig. 7.12a, b), diabetes, and sarcoid neuropathy (Pearson and Weddell 1975; Vallat et al. 1991; Thomas and Bhagat 1978; Nesbitt and Acland 1980; Johnson and Kline 1989). The occasional finding of a few disorganized nerve fibers that invade the perineurium and even epineurium, forming a microneuroma, is a response to focal injury of neural and perineurial elements, and we have on occasion observed this in diabetic neuropathy (Figs. 17.2 and 17.4b).
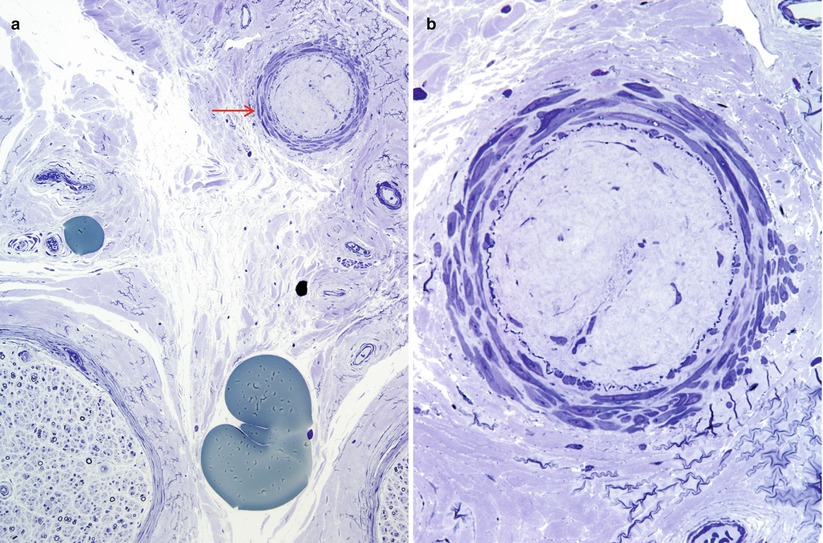

Fig. 7.11
Non diagnostic vascular alterations (a, b): This non-diabetic presented with mononeuropathy multiplex. An epineurial arteriole (arrow, a) shows hyaline luminal obliteration with preservation of internal elastica. Although suggestive this lesion is insufficient for a diagnosis of vasculitis. These were the only vascular alterations seen in the entire specimen. (1 μ thick toluidine blue stained plastic sections [a, 200×; b, 600×])
Lipid-filled cells infiltrating the perineurium can be seen in lepromatous leprosy and were particularly prominent in a case of idiopathic perineuritis we have examined (Fig. 10.7) and have also been described in primary biliary cirrhosis (Thomas and Walker 1965). There may be an association with high serum cholesterol. Perineurial cells may accumulate lipid in Fabry and Niemann–Pick diseases and in the neuropathy seen with amiodarone and other amphiphilic cationic toxins. Polarized light examination of fresh frozen tissue demonstrates a “Maltese cross” pattern in some lipid storage diseases (Fig. 20.10), but this is a nonspecific property of anisotropic cholesterol esters (Weller 1967; Hirsch and Peiffer 1957).
7.4.3 Examination of the Endoneurium
7.4.3.1 Light Microscopy: Characterization of the Neuropathic Process
The pathologist must decide if a neuropathy is present and, if so, identify the process involved.
Is There a Loss of Myelinated Axons? Are Large or Small Fibers Selectively Affected?
With toluidine blue sections, at a magnification of 20×, we achieve a quick estimation of whether there is severe, moderate, mild, or no discernible axon loss. Given the wide range of normal, it is not possible to detect anything less than 25 % axonal depletion, although a fiber frequency–diameter histogram may show a significant alteration in large to small myelinated fiber number ratio even with a normal total myelinated fiber number (see Chap. 3 for additional discussion of quantitative morphometry). Even without morphometry, an impression of the relative proportion of large and small myelinated fiber loss should be obtained, the former being more commonly affected, regardless of etiology. A selective loss of small myelinated fibers (Fig. 7.13a) confirmed by morphometry (Fig. 7.13b) is a finding of considerable importance, with a limited differential diagnosis (Table 7.7). Light microscopy does not readily permit assessment of unmyelinated fiber numbers on routine sections, even under oil immersion, although early workers used silver impregnation to derive good estimates of unmyelinated fiber counts and even diameter (Thomas et al. 1993). Johnson and colleagues (1994) have recently advocated the use of light microcopy with immunocytochemical labeling of unmyelinated axons for rapid estimation of UF density.