And there I stood, a man grown, shaking in the sunshine with that old boyish emotion brought back to me by an odour! … Often and often have I known this strange rekindling of dead fires. And I have thought how, if our senses were really perfect, we might lose nothing out of our lives: neither sights, nor sounds, nor emotions….
I. THE SENSES
A. Sensation and subjectivity
The possibility for sensation begins when a chemical or physical change stimulates the receptor endings of sensory neurons and alters the flow of impulses in the sensory pathways. The impulses in the sensory pathways then lead to an experience that we call a sensation, such as pain, touch, or sight. Nothing is more real to the patient (Pt) than the experience of the sensation, such as pain, nor less real to the observer. Although the Pt can judge the degrees of a sensation, even on scale of 0 to 10 for pain, no one else can verify the sensation or measure it objectively in grams, centimeters, or seconds, the classic units of the physics. Nevertheless, by carefully eliciting the Pt’s history, the examiner (Ex) can recognize and diagnose various sensory syndromes, such as migraine or nerve root compression, with about the same degree of certainty as motor syndromes.
B. Classification of sensation
1. Aristotle recognized five primary senses:
a. Sight
b. Sound
c. Smell
d. Taste
e. Touch
2. Tradition also recognizes special and general senses. The special senses are sight, sound, taste, smell, and equilibrium/verticality. The general sensations are the rest. Sensation also can be classified as somatic or visceral.
3. Charles Sherrington (1857–1952) classified sensation as exteroception, proprioception, and interoception, depending on the origin of the stimulus and the location of the receptor tips of the axons.
a. Exteroceptor axonal tips are located near the external body surfaces. They respond to stimuli that impinge on the body’s external surfaces. These stimuli produce sight, sound, smell, taste, and superficial cutaneous sensation. Superficial skin sensations include:
i. Touch
ii. Superficial pain
iii. Temperature
iv. Itching, tickling, and wetness
b. Proprioceptor axonal tips are located beneath body surfaces. They respond to stimuli that originate from receptors deep in the dermis, in muscles, tendons, ligaments, and the vestibular labyrinth. In large part, they record the actions of the body on itself and orientation to the pull of gravity. (See page 395 for a fuller definition of proprioception.) Proprioceptive sensations include:
i. Position
ii. Movement
iii. Vibration
iv. Pressure, weight, or tension
v. Deep pain (sometimes included in proprioception)
vi. Equilibrium and verticality (via vestibular pathways and dorsal columns)
c. Interoceptor axonal tips located in the viscera and vessels respond to stimuli that act on the internal surfaces of the viscera or originate in the visceral walls:
i. Visceral and vascular pain
ii. Sense of fullness or distention of the viscera
4. Obviously the various sensory classifications overlap and are inconsistent. To obviate memorizing sensory classifications, think systematically and simply sort through your own senses.
C. The concept of sensory modalities
1. No normal person confuses the stench of carrion with a flash of light or a pinprick with a sound. Each unique sensation not resolvable into a more elementary sensation is called a primary sensory modality. Ay, but there is the rub: How does one define unique and elementary? (Synesthesia refers to the condition where one type of stimulation evokes the sensation of another; hearing a sound evokes the visualization of a color.)
2. A sensation felt in one part of the body as a result of stimulus applied to another, as in referred pain.
3. Operations to disclose primary sensory modalities: Stick yourself with a pin: There, that is pain, one modality. Stroke yourself with a piece of cotton: There, that is touch, another modality.
4. Operation to disclose multimodal sensations: Close your eyes and grasp any object, say a quarter, from your pocket or purse. You will recognize it as a metal disc by a combination of touch, texture, weight, size, circularity, and even its slightly cold feel. Thus we can resolve object recognition as a combination of several exteroceptive and proprioceptive modalities. Multimodal sensations include:
a. Form, size, shape, texture, and weight
b. Itching, tickling, and wetness
D. Implications of the theory of modality specificity
1. The neurologist constructs the entire sensory examination on modalities because the pathways of the nervous system provide for modality separation. In fact, we might even define a modality as any sensation that the nervous system represents by a unique pathway, but that requires negative definitions. What is sight? It is that sensation lost after cutting both optic nerves. Each of us has to rely on our private experience to distinguish different modalities.
2. Because unique receptors serve each of the special senses, investigators have sought unique receptors for all modalities. Carried to its extreme, the theory of modality specificity requires unique receptors, unique peripheral axons, unique pathways through the cord, brainstem, and thalamus, and unique cortical receptive areas. This theory of modality-specific pathways enables clinicians to localize lesions, but does not reflect the fact that most stimuli tend to excite all afferents and cortical neurons receive convergent input from multiple afferent fibers (Saal and Bensmaia, 2014; Vriens et al, 2014). For example, the sensations of shape, texture, wetness or vibration represent the integration of cutaneous sensory information that begins at the spinal cord and extends to subcortical and cortical levels (Abraira and Ginty, 2013; Filingeri et al, 2014).
3. By testing all sensations, the Ex tests the integrity of a large volume of neural tissue. Add the volume of tissue assayed by testing motor pathways, and the Ex has tested the integrity of the spinal cord, the brainstem, the cerebellum, and much of the diencephalon and cerebral hemispheres. The more pathways that function normally, the more the Ex can exclude neurologic disease. The more pathways that function abnormally, the more the Ex can predict the size, location, and type of the lesion.
E. Basic principles of sensory physiology
These principles are summarized from the doctrine of specific nerve energies of Johannes Müller (1801–1858):
1. Sensation is an awareness of the state of nerve impulses in the sensory neural pathways. We only know the external world by the changes that occur in the state of impulses in our receptor pathways.
2. Any stimulation of a sensory nerve by any means, electrical, mechanical, or chemical, causes only the type of sensation ordinarily mediated by the nerve. A blow on the eye causes a sensation of light, not taste.
3. The same stimulus applied to different sensory organs causes only the sensation appropriate to the organ. Put a stimulating electrode on the cochlea and you hear. Put the same electrode on the skin and you feel.
II. SMELL (OLFACTION): CRANIAL NERVE I
A. Olfactory receptor and nerve
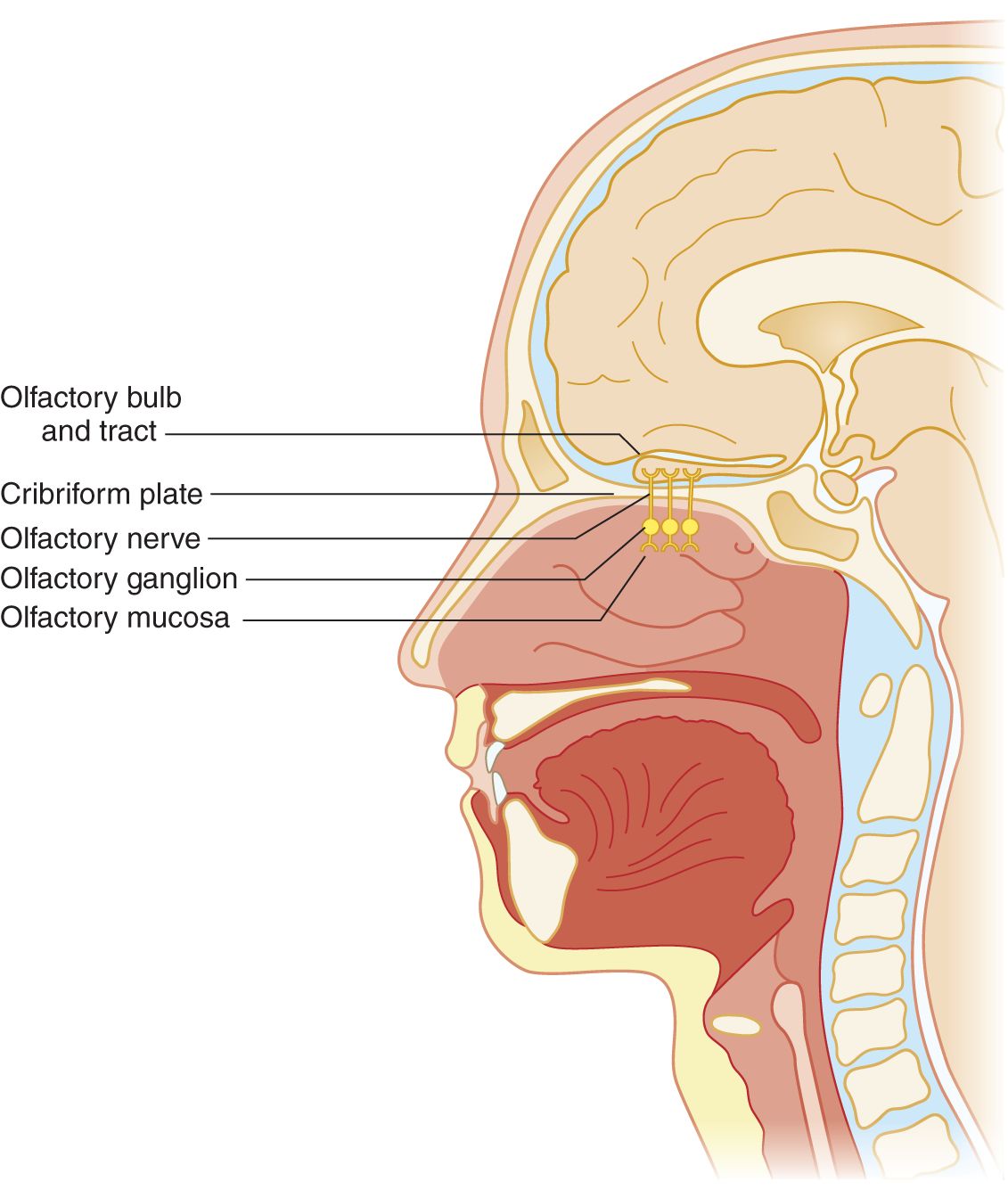
FIGURE 9-1. Sagittal section of head to show olfactory nerve, bulb, and tract.
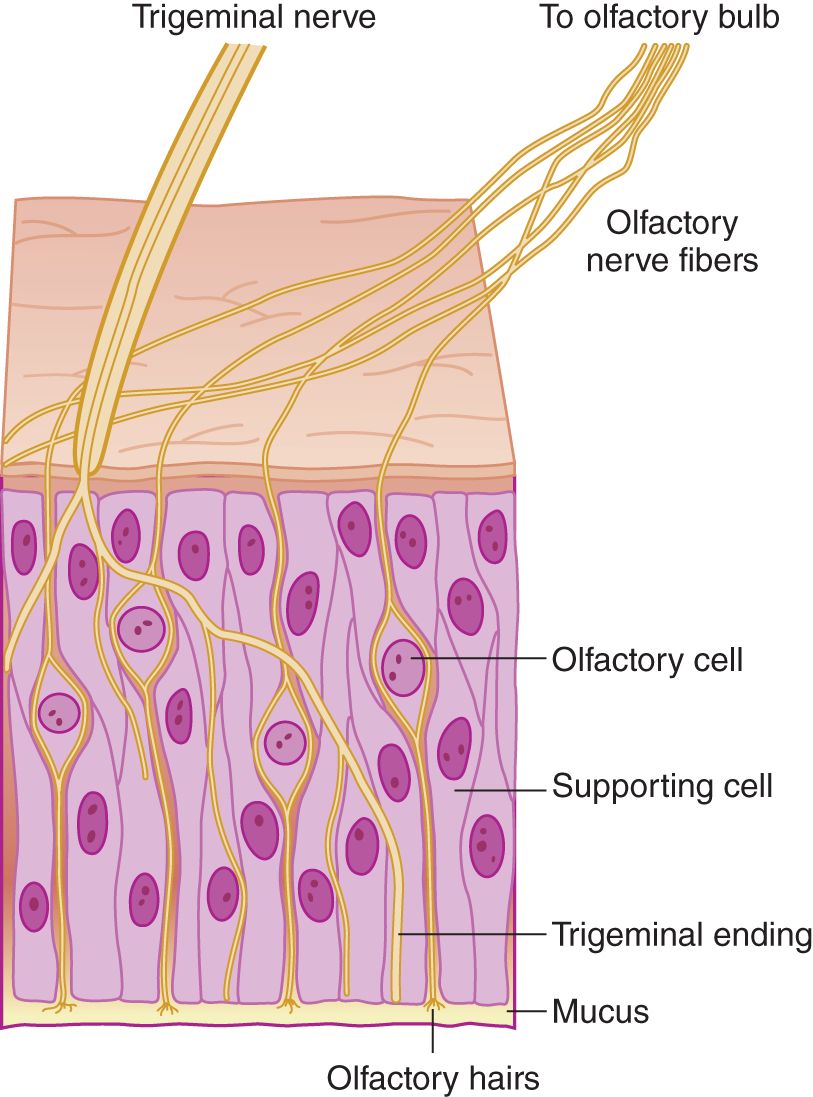
FIGURE 9-2. Microscopic section of olfactory mucosa, showing innervation by cranial nerves I and V. (Reproduced with permission from Amoore JE, Johnston JW, Rubin M. The stereochemical theory of odor. Sci Am. 1964;210:42–49.)
2. Mucus covers the olfactory nerve endings. Any odiferous agent must first dissolve in the mucus, which acts as the first censor for smell. Colds or allergic rhinitis impair olfaction by mechanical reduction of airflow and by excessive mucus secretion, a response triggered in part by these olfactory receptor neurons (Tizzano and Finger, 2013). Hyposmia means partial loss of the sense of smell, and anosmia means complete loss. These olfactory neurons are unique as they are not only the first-order neuron for olfaction, but can regenerate, possess receptors that bind odorants (respond to more than one odorant) and are directly exposed to the external environment.
3. Olfactory impulses travel centrally past the perikarya of the ganglion cells in the nasal mucosa. The ganglion cells are  external to/
external to/ within/
within/ internal to the cribriform plate. (
internal to the cribriform plate. ( external to (Fig. 9-1))
external to (Fig. 9-1))
4. Axons from the olfactory ganglion cells form olfactory nerve filaments. The filaments perforate the cribriform plate and attached dura. The olfactory axons then cross the subarachnoid space to synapse on their second order projection neurons (mitral and tufted cells) within specialized structures (glomerulus) in the olfactory bulbs (Crespo et al, 2013; Lucero, 2013). These second order neurons are the primary efferent projection neurons of the olfactory bulb. Organisms may gain access to the subarachnoid space or brain via the olfactory nerve filaments and cause encephalitis.
B. The olfactory stimulus
1. The two cranial nerves (CrNs) that supply sensory fibers to the olfactory epithelium are _____ and _____. Of these, only CrN _____ serves olfaction. (I; V (Fig. 9-2); I)
2. As a general law in testing any sensation, the Ex isolates the chosen modality from all other modalities. Otherwise, the Ex does not know which sensory pathway caused the response. To test only the sense of smell, should the Ex use an irritating substance such as  ammonia or an aromatic substance such as
ammonia or an aromatic substance such as  coffee? (
coffee? ( coffee)
coffee)
3. Ammonia irritates all receptors of a mucous membrane. Even the conjunctiva reacts to (smells, as it were) ammonia. To test smell, use a vial of coffee grounds. Should the vial be  opaque/
opaque/ transparent? Why? (
transparent? Why? ( opaque)
opaque)
_________
_________
4. Other readily available aromatic substances are oil of lemon, orange peel or apple skin, and soap.
5. Although not used in the routine neurologic examination (NE), there are commercially available smell identification testing kits that incorporate a battery of different odorants to test olfaction (Allis and Leopold, 2012).
C. Technique for testing olfaction
1. Successful sensory testing depends on communication between the Pt and Ex. Say to the Pt, “Close your eyes, sniff, and try to identify this odor.”
2. Compress one of the Pt’s nostrils. Hold the vial in front of the open nostril and ask the Pt to sniff. Wait a moment for the Pt to perceive the odor and then identify it.
3. For the second trial, compress the opposite nostril and this time do not present the stimulus. Withholding the stimulus tests the Pt’s suggestibility and attentiveness. Incorporate such safeguards in all sensory testing.
4. The third time, present the stimulus to the untested nostril.
D. Central olfactory pathways and the concept of a rhinencephalon
1. After receiving the synapses from the primary olfactory axons, the olfactory bulbs send secondary pathways to the adjacent basal frontotemporal junction (basal forebrain). Tertiary pathways then disperse through an array of circuits in the basal forebrain that are not directly accessible to clinical testing but can be imaged (Benarroch, 2010; Demaria and Ngai, 2010; Bekkers and Suzuki, 2013; Lepousez et al, 2013).
2. Taken together, the olfactory bulbs and tracts and their immediate central connections constitute the rhinencephalon. At one evolutionary stage, the cerebrum consisted mostly of rhinencephalon. Ontogenetically and phylogenetically, our own brain retains the primitive rhinencephalic ground plan (Fig. 9-3).
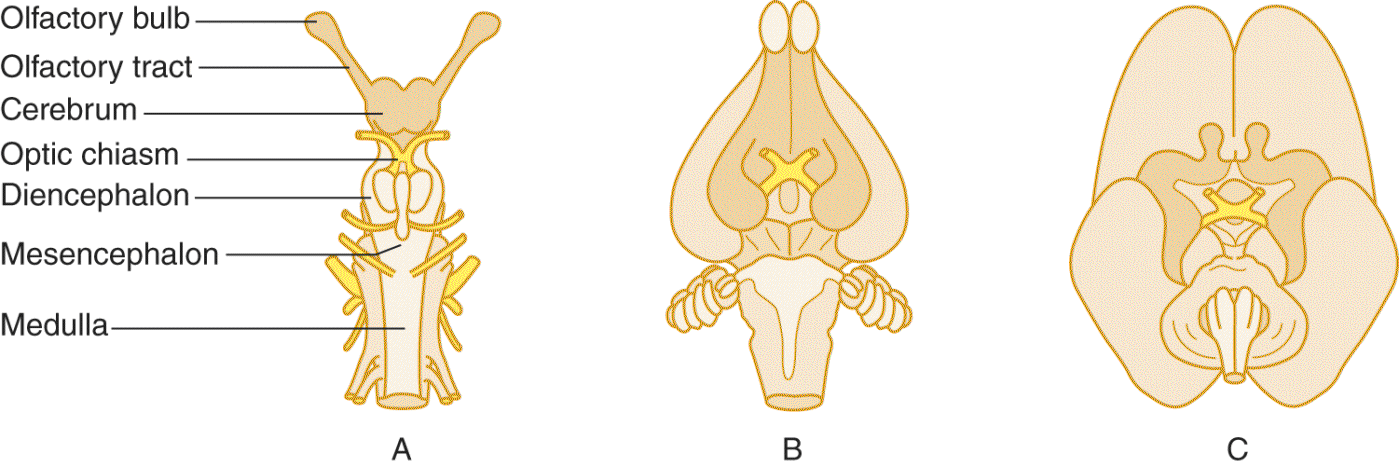
FIGURE 9-3. Ventral views of shark (A), rabbit (B), and fetal human (C) brains. The rhinencephalon (darker tan) comprises most of the shark brain. Notice in the rabbit and human brains that the nonrhinencephalic cortex (unshaded), which began as patches on the cerebral wall of primitive animals, has overgrown to dwarf the rhinencephalon. Nevertheless, the rhinencephalon set its imprint forever on the form and function of the human brain.
3. The sense of smell originally served the two fundamental functions of feeding and mating. These two visceral drives and their attendant visceral emotions were originally localized in the rhinencephalon before extending to those parts of the forebrain, essentially the limbic lobe that evolved most directly from the olfactory ground plan. These forebrain derivatives remain the “seats” of emotion and affective experience. Humans are not believed to exude pheromones which serve to activate neural circuits within the limbic system that result in specific behaviors or regulate hormonal levels (Liberles, 2014), but we assiduously replace them with perfumes and colognes so, perhaps they still play a role (Semin and de Groot, 2013). In any event, smell remains as the most evocative of sensations.
4. Déjà vu and déjà pensée: The uncus, the medial-most gyrus of the temporal lobe, contains a cortical area for smell. Uncal lesions cause olfactory hallucinations, usually of very disagreeable odors. One of my Pts tore down his bedroom walls because of the conviction that he smelled a dead animal entrapped within them. Each time the odor came powerfully to him, he also experienced a peculiar feeling of familiarity, of something happening that had happened before (just as Ray Shannard Baker described). Autopsy showed a metastatic bronchogenic carcinoma in his uncus. The feeling of familiarity, as if something had happened before, is called déjà vu (previously or already seen) or déjà pensée (previously or already thought). Although we each experience this sense of undue familiarity from time to time, when a Pt reports it in association with an olfactory hallucination, suspect a medial temporal lobe lesion. Get a magnetic resonance imaging (MRI) scan.
E. Olfactory-related consequences of head injuries
1. Head injuries may shear off the delicate olfactory nerve filaments, resulting in anosmia (Reiter et al, 2004) and compared to impaired olfaction following upper respiratory tract infections, recovery is infrequent and no clear medical interventions currently exist (Reden et al, 2006, 2012). If the wafer-thin cribriform plate fractures, the meninges may rupture initially or later when the Pt coughs, causing a fistula that allows cerebrospinal fluid (CSF) to gush into the nose. During physiologic fluctuations in intracranial pressure, fluid then refluxes back through the fistula into the subarachnoid space, introducing nasal organisms and causing meningitis or encephalitis. Therefore, consider a CSF fistula in the differential diagnosis of a runny nose (rhinorrhea). Rhinorrhea may occur intermittently and often increases upon bending forward or following the Valsalva maneuver. Suspect such a fistula whenever a Pt, usually one with a history of head injury, has a runny nose and anosmia but does not have a cold or allergic rhinitis. Persistent CSF fistulas at any level of the neuraxis require surgical closure (Prosser et al, 2011; Ziu et al, 2012).
2. To differentiate a CSF leak from nasal mucus or allergic rhinorrhea, a sample of the fluid is tested for β2-transferrin (B2Tr) produced in the brain by neuraminidase activity which is specific for CSF. While results of glucose (higher in CSF then in nasal secretions) were once used as a diagnostic test, current assays are too sensitive and hence often false positive; total protein content, and chloride are not specific for CSF. Technical expertise and delay in obtaining results for B2Tr led to the development of an assay for β-trace protein (present in high concentrations within the CSF) that is as sensitive as B2Tr, but for various reasons, it has yet replaced B2Tr as a diagnostic test. To localize the fistula the nasal cavity is inspected carefully with a speculum and endoscopy, but further diagnostic imaging studies (eg, high-resolution thin section computed tomography, MRI, MR cisternogram, or radionuclide cisternography) are necessary (Ziu et al, 2012); Fig. 9-4 provides a diagnostic algorithm.
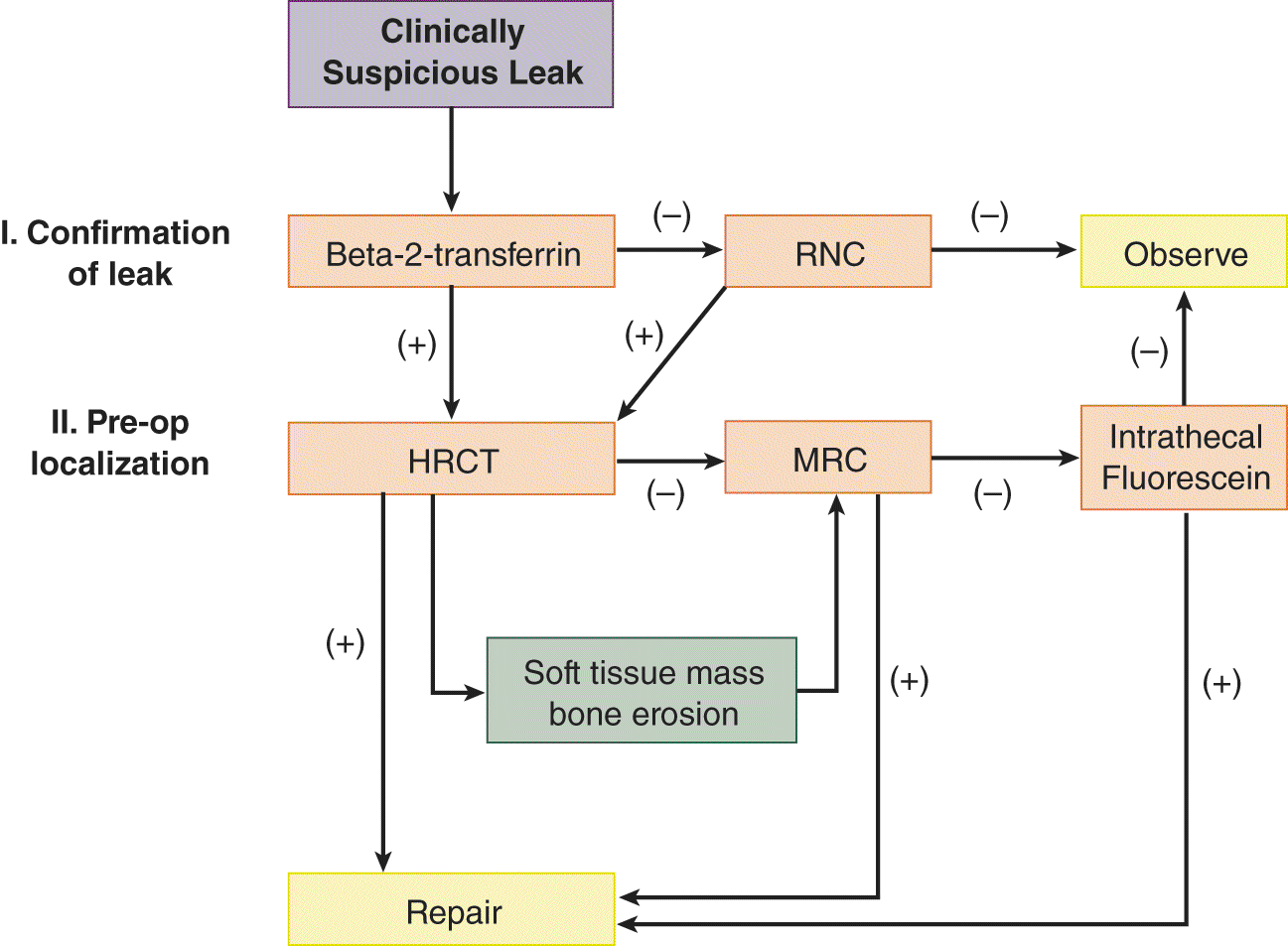
FIGURE 9-4. Diagnostic algorithm for diagnosis and management of suspected skull base cerebrospinal fluid fistulas. HRCT = high-resolution computed tomography; MRC = magnetic resonance cisternography; RNC = radionuclide cisternography. (Reproduced with permission from Zapalac JS, Marple BF, Schwade ND. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;676:669–676.)
3. Some nasal complications of a head injury are
a. Loss of smell, a condition called _________
b. The formation of a fistula between the nasal cavity and the _________
c. A potentially lethal complication of such a fistula is _________
F. Differential diagnosis of anosmia
1. To analyze anosmia systematically, start at the receptor. What initial barrier must any aromatic agent in the inspired air pass through before it stimulates olfactory receptors? _________
a. The most frequent causes of anosmia are the common cold, allergic rhinitis, smoking, and head trauma (Allis and Leopold, 2012).
b. Sadly, aging diminishes the sensitivity of all sensations—sight, hearing, vibration sense, and so on. However, the origin of the age-related impairment of olfaction remains unclear and may have as much of a cortical as a peripheral origin (Mobley et al, 2014). Hyposmia has many etiologies and can accompany various endocrine disorders (eg, hypothyroidism, pseudohypoparathyroidism), meningitis, subarachnoid hemorrhage, local mechanical injury to the olfactory epithelium, medications, psychiatric disorders, Alzheimer and Parkinson disease (Greebe et al, 2009; Moman et al, 2009, Doty, 2012; Schecklmann et al, 2013; Schofield et al, 2014) and therefore requires a careful history to limit the differential diagnosis.
2. Next, consider lesions of the olfactory bulbs and tracts. Although rare, the most significant are meningeal neoplasms—classically, olfactory groove meningiomas—that compress the olfactory bulbs and tracts (Adappa et al, 2011; Jang et al, 2013). The olfactory bulbs and tracts may fail to evaginate (arhinencephaly), resulting in congenital lifelong anosmia (Assouline et al, 1998; DeMyer, 1987) or part of a clinical syndrome where the individual may never remember being able to smell (Karstensen and Tommercup, 2012).
3. Figure 9-5 reviews the differential diagnosis of anosmia.
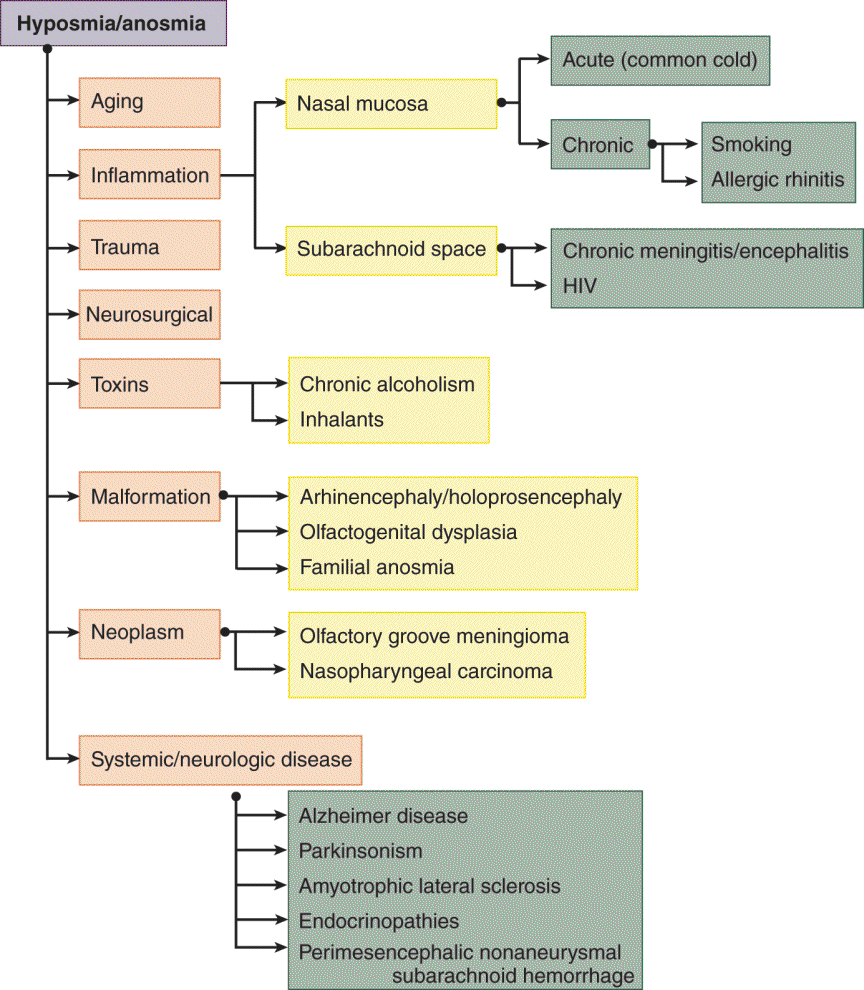
FIGURE 9-5. Dendrogram for the differential diagnosis of hyposmia and anosmia (reference).
4. What is the explanation for nasal drip caused by sneezing or coughing after a head injury?
_________
_________
5. The Pt with anosmia may complain mainly of loss of taste, because taste and smell are so intimately linked and quality of life often suffers with their impairment (Croy et al, 2014).
BIBLIOGRAPHY · Sensation
Abraira VE, Ginty DD. The Sensory Neurons of Touch. Neuron. 2013;79:618–639.
Filingeri D, Fournet D, Hodder S, Havenith G. Why wet feels wet? A neurophysiological model of human cutaneous wetness sensitivity. J Neurophysiol. 2014;112:1457–1469.
Saal HP, Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci. 2014;37:689–697.
Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–589.
Smell
Adappa ND, Lee JYK, Chiu AG, Palmer JN. Olfactory Groove Meningioma. Otolaryngol Clin N Am. 2011;44:965–980.
Allis TJ, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin N Am. 2012;20:93–111.
Assouline S, Shevell MI, Zatorre RJ, et al. Children who can’t smell the coffee: isolated congenital anosmia. J Child Neurol. 1998;13:168–172.
Baker RS (David Grayson, pseudonym). Adventures in Contentment. New York, NY: Grossett; 1907.
Bekkers JM, Suzuki N. Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci. 2013;36:429–438.
Benarroch EE. Olfactory system; functional organization and involvement in neurodegenerative disease. Neurology. 2010;75:1104–1109.
Crespo C, Liberia T, Blasco-Ibáñez JM, et al. The circuits of the olfactory bulb. The exception as a rule. Anat Rec. 2013;296:1401–1412.
Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses. 2014;39:185–194.
DeMaria S, Ngai J. The cell biology of smell. J Cell Biol. 2010;191:443–452.
DeMyer W. Holoprosencephaly (cyclopia-arhinencephaly). In: Vinken PJ, Bruyn GW, Klawans HL, eds. Malformations. Handbook of Clinical Neurology. Vol 6. Amsterdam: Elsevier Science; 1987, Chapter 13, pp. 225–244.
Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis. 2012;46:527–552.
Greebe P, Rinkel GJE, Algra A. Anosmia after perimesencephalic nonaneursmal hemorrhage. Stroke. 2009;40:2885–2886.
Jang WY, Jung S, Jung TY, et al. Preservation of olfaction in surgery of olfactory groove meningiomas. Clin Neur Neurosurg. 2013;115:1288–1292.
Karstensen HG, Tommerup N. Isolated and syndromic forms of congenital anosmia. Clin Genet. 2012;81:210–215.
Lepousez G, Valley MT, Lledo PM. The impact of adult neurogeneses on olfactory bulb circuits and computations. Annu Rev Physiol. 2013;75:339–363.
Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151–175.
Lucero MT. Peripheral modulation of smell: fact of fiction? Sem Cell Dev Biol. 2013;24:58–70.
Mobley AS, Rodriguez-Gill DJ, Imamura F, Greer CA. Aging in the olfactory system. Trends Neurosci. 2014;37:77–84.
Moman MR, Verweij BH, Buwalda J, Rinkel GJL. Anosmia after endovascular and open surgical treatment of intracranial aneurysms. J Neurosurg. 2009;110(3):482–486.
Prosser JD, Vender JR, Solares CA. Traumatic Cerebrospinal Fluid Leaks. Otolaryngol Clin N Am. 2011;44:857–873.
Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132:265–269.
Reiter ER, DiNardo LJ, Costanzo RM. Effects of head injury on olfaction and taste. Otolaryngol Clin N Am. 2004;37:1167–1184.
Schecklmann M, Schwenck C, Taurines R, et al. A systematic review on olfaction in child and adolescent psychiatric disorders. J Neural Transm. 2013;120:121–130.
Schofield PW, Finnie S, Yong YM. The role of the olfactory challenge tests in incipient dementia and clinical trial design. Curr Neurol Neurosurg Rep. 2014;14:479.
Semin GR, de Groot JHB. The chemical bases of human sociality. Trends Cogn Sci. 2013;17:427–429.
Tizzano M, Finger TE. Chemosensors in the nose: Guardians of the airways. Physiology. 2013;28:51–60.
Warnecke A, Averbeck T, Wurster U, et al. Diagnostic relevance of beta-2 transferrin for the detection of cerebrospinal fluid fistulas. Arch Otolaryngol Head Neck Surg. 2004;130:1178–1184.
Zapalac JS, Marple BF, Schwade ND. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;126:660–676.
Ziu M, Savage JG, Jimenez DF. Diagnosis and treatment of cerebrospinal fluid rhinorrhea following accidental traumatic anterior skull base fractures. Neurosurg Focus. 2012;32:E3.
III. TASTE (GUSTATION) AND LOSS OF TASTE (AGEUSIA)
A. Receptors
The epithelium of the tongue and tonsillar pillars contains taste buds (fungiform and circumvallate papillae; Roper, 2013) where specific receptors detect different taste modalities, but these same receptors have been identified on nontaste tissues where they may exert metabolic roles (Li, 2013; Laffitte et al, 2014). As in olfaction, the chemical agents that stimulate taste must first dissolve in a liquid, the saliva. Loss of taste is called ageusia. Often the Pt who complains of ageusia actually has anosmia, because taste and smell complement each other in producing flavor and full gustatory sensation (Allis and Leopold, 2012). Hypogeusia may be present in up to 5% of the population, complete ageusia seems to be rare, but misinterpretations or categorization of tastes common (Welge-Lüssen et al, 2011). When pathologic changes in taste buds were found in patients with the syndrome of idiopathic hypogeusia with dysgeusia, hyposmia, and dysosmia (Henkin et al, 1971) it was later attributed to reduced total serum zinc levels. As hypogeusia was linked to zinc deficiency in humans, it was the rationale for related tests (and supplementation), but currently none seem to be sensitive and specific with regards to detecting marginal zinc levels (Gruner and Arthur, 2012).
B. Innervation of taste receptors
1. The taste buds of the anterior two-thirds of the tongue are innervated by … which CrN was it? Well, if you have forgotten, start at CrN I and sort through them:
a. CrN _______ smells, and _______ sees. (I; II)
b. CrNs _______, _______, and _______ rotate the eyeball. (III; IV; VI)
c. CrN _______ chews and feels the front of the head. (V)
d. CrN _______ moves the facial muscles, tears, snots, salivates, and _________
2. To test taste, use the anterior two-thirds of the tongue, the area innervated by CrN VII, because of the inconvenience of reaching the taste buds on the posterior third of the tongue and tonsillar pillars. Review Figs. 6-5 and 6-6. (VII; tastes (Should you review the brief description of the CrNs in Tables 2-5 and 2-6?))
3. In contrast to earlier maps, the tongue does not show clinically significant regional differences to salty, sweet, sour, and bitter tastes and there exists a great deal of overlap between each taste modality (Breslin, 2013). (There is also a visual aspect to eating and the color of foods can also inform, but not always indicate their nutritional value; Barnes et al, 2013).
C. Review of cranial nerve VII
1. CrN VII attaches to the brainstem at the _________
2. In ventrodorsal order, the CrNs attached to the pontomedullary sulcus are _________
3. CrN VII enters the internal auditory meatus in company with CrN _________
4. The primary neurons for taste occupy the only ganglion on CrN VII, the _________
5. The name geniculate ganglion comes from the knee-like downward bend of CrN VII after it clears the ganglion and heads for the stylomastoid foramen (Figs. 6-5 and 6-6).
D. Central pathways for taste
Lesions of the central taste pathways rarely cause isolated loss of taste, but often hypogeusia. The brainstem pathway ascends ipsilaterally within the central tegmental tract in the lateral part of the medulla and tegmentum of the pons arising from the nucleus of the tractus solitarius where the taste fibers from CrNs VII, IX, and X converge. (There is evidence that distinct taste modalities are transmitted via segregated pathways to the brain; Carleton et al, 2010). It appears these fibers cross (all or some?) at the midbrain so, lesions inferiorly will usually cause an ipsilateral deficit (Combarros et al, 2000), those superior contralateral, but a bilateral deficit assumed to reflect involvement of both crossed and uncrossed fibers. These fibers terminate in the most medial portion of the ventromedial nucleus of the thalamus and project to the insular cortex (island of Reil) and adjacent parietal operculum (Landis et al, 2006; Nakajima et al, 2010; Tsivgoulis et al, 2011; Maffei et al, 2012). Since an initial description of irritative lesions in this cortical region causing gustatory hallucinations (Penfield and Jasper, 1954), while cortical lesions typically cause bilateral hypogeusia or hemiageusia of which the Pt is unaware (Landis et al, 2006).
E. Technique of testing for loss of taste (ageusia)
1. Stimulus: The stimulus is a salty, sweet, sour, or bitter substance. Table salt, sugar, or quinine is suitable. Conceal the salt or sugar. The Pt who sees a white crystalline substance will almost automatically guess salt or sugar.
2. Communication with the patient
a. Tell the Pt, “I want to place something on your tongue for you to taste. Stick out your tongue and keep it out. When you recognize the taste, hold up your hand.”
b. Place a few crystals of your test material on the right or left half of the tongue and massage these around with the well-moistened cotton tip of an applicator stick. Take care to confine the stimulus to one-half of the tongue. Do not allow the Pt to return the tongue to the mouth because the saliva will diffuse the taste stimulus beyond the area selected for testing. If the tongue is dry, moisten it slightly. Allow 15 to 20 seconds for the substance to dissolve and for the Pt to respond. Some normal subjects will not perceive sugar. Test again with salt.
c. After the Pt rinses his mouth, test the opposite side of the tongue with the same or a different substance. For routine clinical purposes you need try only one substance to test for ageusia. Although not part of the routine NE, taste can be measured by impregnated paper discs and by galvanic currents.
d. Test your own sense of taste as described.
e. The main indications for testing taste are a complaint of loss of appetite, smell, or taste or the presence of CrN VII palsy. Taste is the only clinically testable sensation mediated by CrN VII. Patients may lose taste or suffer a perversion of taste (dysgeusia) because of various medications, systemic illness, cancer, and endocrinopathies (Allis and Leopold, 2012; Mennella et al, 2013).
F. Clinical value of testing taste in facial palsy
1. Patient protocol: A 26-year-old woman awoke one morning with her face “drawn to one side.” Examination disclosed that on the left side she could not wrinkle her forehead, close her eye, pull back the corner of her mouth, or wrinkle the skin of her neck. She moved the right side of her face normally. Her complaint of “drawing” of her face was due to the unopposed pull of the intact right-side facial muscles, which pulled her lips to the right when she spoke or smiled (see the Pt in Fig. 6-7). The remainder of the examination was completely normal, including taste sensation and hearing.
2. Analysis of the clinical data will lead to a conclusion as to where and what the CrN VII lesion is.
a. In analyzing a motor deficit, consider first its distribution. Does it match a central or a pyramidal tract (upper motoneuron) distribution? Does it match a root or peripheral nerve or myopathic distribution?
b. Which distribution does the motor deficit of the present Pt match?  upper motoneuron/
upper motoneuron/ peripheral nerve/
peripheral nerve/ myopathic. (
myopathic. ( peripheral nerve)
peripheral nerve)
c. The paralysis involves the muscles of one nerve, CrN _________
d. The distribution of the paralysis in the field of one nerve excludes a neuromyal junction disorder or myopathy; these are widespread disorders and not limited to a single nerve.
e. Because interruption of a single nerve explains the paralysis, the disorder consists of a  mononeuropathy/
mononeuropathy/ polyneuropathy/
polyneuropathy/ myopathy. (
myopathy. ( mononeuropathy)
mononeuropathy)
3. Having identified a mononeuropathy of CrN VII, we have to specify the location of the lesion along the course of the nerve. In analyzing a sensory disturbance or a reflex arc, we invoked the principle of starting at the _________
4. Where should you start to trace along the course of the impulses in a motor nerve? _________
5. The CrN VII nucleus occupies the  tectum/
tectum/ tegmentum/
tegmentum/ basis of the
basis of the  midbrain/
midbrain/ pons/
pons/ medulla. (
medulla. ( tegmentum;
tegmentum;  pons)
pons)
6. Because of the close packing of tracts and nuclei, a brainstem lesion would rarely affect just one CrN nucleus. It most likely would involve the neighboring lemniscal, cerebellar, or CrN VIII pathways or neighboring CrNs. In addition to VII, the CrN motor nuclei in the pons are _________
7. The Pt had no signs implicating structures in the vicinity of the CrN VII nucleus in the central nervous system (CNS); therefore, the lesion most likely interrupted the nerve  inside/
inside/ outside the brainstem. (
outside the brainstem. ( outside)
outside)
8. After leaving the pontomedullary sulcus of the brainstem and before entering the internal auditory meatus, CrN VII must cross the _________
9. The subarachnoid space between the cerebellum and the brainstem is called the cerebellopontine angle. A lesion here, such as a neoplasm, would interrupt not only CrN VII, but also CrN ______. (VIII)
10. As the neoplasm enlarged, in addition to CrNs VII and VIII, it would affect CrNs _________
11. Go to Fig. 2-20 to appreciate how a relatively common tumor, an acoustic neuroma, in the cerebellopontine angle can affect additional nerves. Start with your pencil on CrN VIII as the center and shade in a circle about 1 to 1.5 cm in diameter to see how the lesion would encroach on adjacent nerves and the brainstem as it grows.
12. If the lesion occupied the internal auditory meatus or canal, other than CrN VII, which CrN would it affect? _________
13. If the lesion interrupted the trunk of CrN VII somewhere between its point of exit from the brainstem and the geniculate ganglion, the Pt should have lost _________
14. CrN VII innervates one muscle in the middle ear, the _________
15. Because the Pt retained taste and had no hyperacusis, the CrN VII lesion must be / outside distal to/
outside distal to/ outside within the middle ear. (
outside within the middle ear. ( distal to)
distal to)
16. If distal to the middle ear, the lesion might be in the facial canal, but a lesion deep within the canal in the temporal bone bars direct clinical examination. If a lesion interrupted CrN VII after its exit from the stylomastoid foramen, the Ex should find pain or swelling in the parotid region, as from an inflammatory or neoplastic mass, but the Pt had no mass or pain in the parotid region.
17. The next link in a motor nerve comes at the terminal tips of its axons, where the axons synapse on the muscles, a region called the _________
18. Explain why the Pt’s lesion is not at the neuromyal junction or in the muscle itself._________
_________
19. Make a line across Fig. 6-5 at the most likely site of the Pt’s lesion.
20. The Pt had idiopathic facial paralysis (Bell palsy), the most common etiology for this common mononeuropathy of CrN VII. The lesion is usually an inflammation, frequently caused by viruses (particularly herpes simplex virus 1) and resulting in either demyelination along the course of the nerve or as the facial nerve swells, compression within the facial canal (de Almeida et al, 2014; Zandian et al, 2014). The Pt recovered good facial function by 6 weeks after onset and a typical outcome for 60% to 90% of Pts, but aberrant regeneration of CrN VII can lead to synkinetic movements (innervation of inappropriate muscles resulting in involuntary movements) or reinnervation of lacrimal rather than salivary glands (resulting in the phenomena of “Crocodile tears” manifested as tearing when eating). EMG can offer prognostic information early in the course and also contribute to clinical management decisions (Mancini et al, 2014; Schwartz et al, 2014). (Your line should cross the facial canal distal to the chorda tympani nerve but proximal to the stylomastoid foramen.)
21. This Pt shows how testing taste helps to localize a lesion along the course of CrN VII. Unless the Pt’s symptoms and signs implicate taste, smell, or CrN VII, you may omit taste testing; but make the omission by discretion, not carelessness.
BIBLIOGRAPHY · Taste Testing and Bell Palsy
Allis TJ, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin N Am. 2012;20:93–111.
Barnes S, Prasain J, Kim H. In nutrition, can we “see” what is good for us? Adv Nutr. 2013;4:327S–334S.
Breslin PAS. An evolutionary perspective on food and human taste. Curr Biol. 2013;23:R409–R418.
Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


