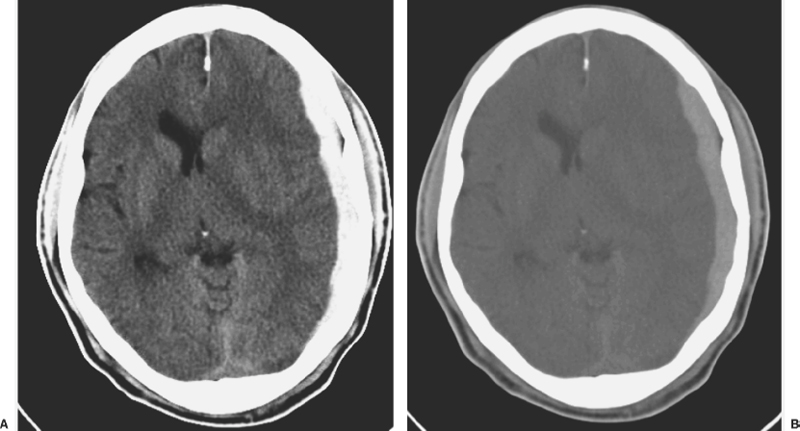
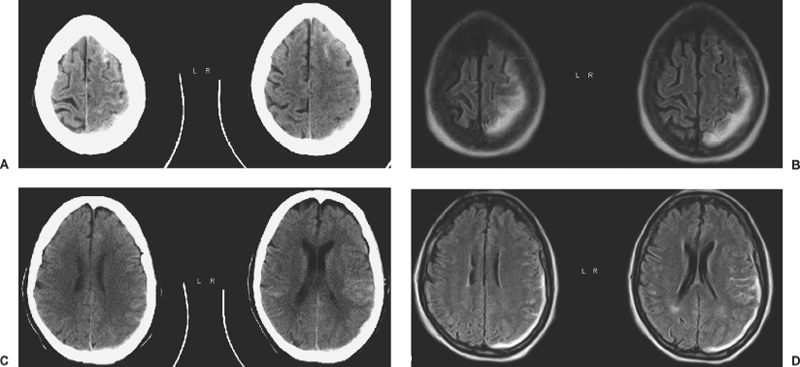
6 Extra-Axial Hematomas Shelly D. Timmons The term “extra-axial hematomas” is used to refer to hematomas found within the intracranial space but outside the substance of the brain itself. These lesions are among the most common emergencies encountered in neurosurgical practice, and almost always occur as a result of head trauma. Head injury is the most important contributor to mortality and morbidity from trauma,1 which is the leading cause of death for people under 45 years of age.2 Rapid evacuation of extra-axial hematomas is the mainstay of neurosurgical intervention for traumatic brain injury (TBI), and timely surgical intervention improves functional outcome and reduces mortality.3 For TBI patients who are initially lucid and deteriorate, four of five will have a mass lesion potentially requiring surgical evacuation, half of which are extra-axial.4 Most patients who present with signs of uncal or transtentorial herniation after trauma have extra-axial mass lesions,5,6 and evacuation can reverse the brainstem signs once decompression is achieved surgically. The entities to be discussed include acute epidural hematoma (EDH), acute subdural hematoma (aSDH), subdural hygroma, subacute subdural hematoma (sSDH), and chronic subdural hematoma (cSDH). Special considerations of posterior fossa (PF) lesions and child abuse are also presented. Figure 6-1 A depiction of a large left temporal epidural hematoma with effacement of the quadrigeminal cisterns from temporal lobe displacement. Epidural hematoma (Fig. 6-1) is a relatively unusual occurrence and is most often caused by trauma, although rare instances of spontaneous occurrences in special circumstances, for example, sickle cell disease, have been reported. The overall incidence among trauma patients is estimated at between 2.7% and 4.1% of TBI patients7,8; however, the incidence among trauma victims in coma is higher, at 9% to 15%.9,10 About 1% of TBI patients with normal neurological examination and cranial fractures and 9% of patients in coma with fracture harbor an EDH.9,11 Epidural hematoma in children is associated with fracture ~40% of the time, half of which are depressed, whereas fractures are uncommon with aSDHs in children.12 Traumatic EDH occurs most frequently in young people following high-speed accidents. In young adults the highest incidence is between 20 and 30 years of age, and above 60 years of age, EDH is unusual,7,12,20 probably due to increasing adherence of the dura mater to the inner surface of the skull. In children, peak incidence is between 5 and12 years, being much less common in newborns and young children.13 Motor vehicle accidents (MVAs) account for the majority of etiologies in patients of all ages (30% to 73%), followed by falls (7% to 52%), and assault (1% to 19%).14 Falls are more commonly the cause of EDH in children than are MVAs,13,15 and children are less likely to present unconscious, to require surgery, or to have associated intracranial lesions.15 Isolated EDHs in children are also predominantly caused by falls (68.6% in one series).16 Multiple (including bilateral) EDHs can occur,8,17,18 typically in the frontal region, and are most often associated with altered sensorium without lucid interval8 (Fig. 6-2). These injuries have also been associated with lower Glasgow Coma Scale (GCS) score on presentation and higher mortality.17 Epidural hematoma can extend both above and below the tentorium cerebelli. This finding may be associated with a venous sinus injury; therefore surgical evacuation may be quite perilous. Chronic EDHs are generally smaller, more likely frontal or parietal, and present with milder, less-specific symptoms; they have excellent outcomes, whether treated operatively or observed.18 Figure 6-2 Bilateral temporal epidural hematoma with underlying right temporal contusion/intraparenchymal hemorrhage. A well-described cause of EDH is a blow to the temporal region resulting in a fracture of the squamous portion of the temporal bone, with subsequent injury to the middle meningeal artery as it travels through or exits the bone. This results in arterial bleeding into the epidural space of the temporal fossa. However, recent evidence suggests that venous sources of EDH are actually more common.19 This may be due to more frequent diagnoses being made possible by widespread rapid availability of computed tomographic (CT) scanning, even in less symptomatic cases. Most EDHs are stable in size soon after trauma, as it is thought that once the dura is sheared from the inner surface of the pericranium and fills with hematoma, the cavity does not continue to expand.18,20,21 Epidural hematomas that are potentially less rapidly expansive may be caused by a tear of a dural sinus, with or without associated overlying fracture. Tears of the bridging veins of the epidural space or diploic veins may also cause EDH. Finally, EDH is often associated with orbitofacial fractures, such as orbital roof or frontal sinus fractures, and may result from direct bleeding from bone or an associated vascular injury. Epidural hematoma can occur intraoperatively as a result of decompression of a contralateral lesion, and needs to be considered whenever intraoperative swelling or post-operative intracerebral pressure become uncontrollable. Late EDH can occur, often as a result of assault, and the outcome is generally more favorable.18 Epidural hematomas are more likely than aSDHs to expand or to appear as new lesions on early follow-up CT, but have also been shown to spontaneously reabsorb on early follow-up CT.22,23 The classically defined “lucid interval” actually occurs in a minority of patients.7,12,13,15,24–27 The lucid interval occurs following a blow to the head, with or without altered sensorium. There follows a period of time, up to approximately 30 minutes, in which blood is accumulating in the extradural space, but the brain is not compressed enough to cause alteration in consciousness. When the mass effect becomes significant, brain compression and transtentorial herniation resulting in rapid loss of consciousness (and even death if untreated) can occur. The most common locations of surgically evacuated EDH are the temporal and temporoparietal regions.7,12,13,25,28 When EDH occurs in the temporal fossa, a relatively small intracranial space, transtentorial herniation can occur rapidly via compression of the temporal lobe and compression of the brainstem. Patients presenting in coma or deteriorating to coma preoperatively comprise 22% to 56% of EDH patients; another 12% to 42% remain conscious until surgery.14 Other presenting findings include nausea and vomiting, hemiparesis or hemiplegia, dysphasia or aphasia, posturing, and seizure activity. Pupillary abnormalities occur in 18% to 44%, but a significant proportion of patients (3% to 27%) present neurologically intact.14,29,30 Even asymptomatic patients exhibiting minor head trauma can harbor EDH diagnosed only with CT scan.31,32 Children with isolated EDH often present with nonspecific findings of headache, vomiting, or not feeling well, leading to frequent delays in diagnosis.13,16 CT scanning is the diagnostic method of choice. Since it has come into routine usage over the past three decades, its ready availability, rapid scanning capacity, and detailed resolution provide quick and accurate diagnosis of not only the size, extent, and location of the EDH, but also of overlying skull fractures when present. Axial imaging can, however, exclude small fractures, especially if they are parallel to the scanning plane, so close inspection of the scout view is warranted. Skull X-rays do not aid in diagnosis, as ~35% are interpreted as normal,29 although, especially for patients with minor head injuries, if a fracture is identified on plain film, CT is recommended due to the high incidence of EDH in these patients.31 The typical CT appearance of an EDH is a hyperdense biconvex extra-axial lesion that does not cross the bony sutures. (Fig. 6-1 and Fig. 6-3). There may be associated relatively hypodense (either isodense or hypodense to brain) areas within the body of the hematoma suggesting a “hyperacute” or swirling blood component, thought to indicate active bleeding into the hematoma or areas of liquid blood associated with coagulopathy.33,34 Occasionally air bubbles will be present within the hematoma (22.5% to 37.0%), although there is no known correlation with outcome35 (Fig. 6-4). Most EDHs are not associated with significant underlying brain injury, and postoperatively the appearance of the brain on CT quickly returns to normal.36 Left surgically untreated, however, EDHs identified on “ultraearly” CT (<3 hours) scans do tend to enlarge, so repeat CT within 12 hours is recommended.22 Figure 6-3 A large left hemispheric epidural hematoma with left temporal contusion (arrow, left panel), seen on coronal CT scan. Note the overlying fractures and contralateral fracture on the bone window (arrows, right panel). Figure 6-4 A small bubble of air in a right temporal epidural hematoma with an underlying contusion. Craniotomy provides the most definitive form of surgical treatment of EDH. Patient selection for these lesions that, in the CT era, have been recognized more often in asymptomatic patients, is critical. For individuals presenting in extremis, with altered level of consciousness, or neurological deficit, the decision to operate is more straightforward. Recently, attempts to make surgical recommendations based upon CT characteristics have been made. One group evaluated CT criteria for surgical evacuation retrospectively in a series of 33 children with EDH and found that EDH thickness >18 mm, midline shift >4 mm, moderate or severe mass effect, and location predicted surgery in 31 of 33 patients.37 Recent evidence-based guidelines have recommended evacuation of EDH for lesions greater than 30 cm3 in volume, and that any patient with an EDH and coma (GCS <9) or anisocoria undergo evacuation as emergently as possible.14 These authors indicated that an EDH <30 cm3 and <15 mm thick with <5 mm midline shift in patients with GCS >8 and no focal deficit might be watched, with frequent serial CT and intensive observation in a neurosurgical hospital where operative therapy is immediately available, should the patient deteriorate or the radiograph worsen. Burr hole drainage as a temporizing measure has been advocated by some, but has been shown to be inadequate in clinical use. Emergency burr hole exploration in one consecutive series of 100 patients prior to CT scanning resulted in a high incidence of negative exploration (44) with 6 negative explorations, despite the presence of extra-axial hematoma (4 unilateral aSDH, 1 unilateral EDH, 1 contralateral aSDH).5 Burr hole drainage prior to transfer to an institution where definitive decompression and drainage can be done via craniotomy can result in incomplete evacuation or failure to control bleeding, and may unnecessarily delay care and increase time to decompression.38,39 Burr hole placement and irrigation of EDH in the emergency department may, however, be life-saving if surgery is not immediately available, and results for burr hole drainage for EDH are generally better than for aSDH.40 Craniotomy for evacuation of EDH mandates identification and elimination of the source(s) of bleeding, via cauterization of vessels, waxing of bone sources, etc. Dural sinus lacerations must sometimes be repaired, plugged, or tamponaded. Epidural tack-up sutures are placed in the perimeter and the center of the craniotomy, to prevent subsequent reaccumulation of blood in the epidural space. Bone flaps may usually be replaced due to frequent absence of underlying lesions and edema, and fractures may need to be repaired. Occasionally slit durostomy to ensure the absence of aSDH is employed. In adult patients, nonoperative management may sometimes be safely employed in those EDHs with <10 to 15 mm thickness or <30 cm3 hematoma volume or <5 mm midline shift, and minimal clinical symptoms, including good GCS and normal pupillary examination.14,23,29,41 Conservative management mandates close clinical observation and frequent radiological follow-up.42,50 Resolution of EDH typically occurs over several weeks (3 to 15 weeks),21 but rarely may resolve spontaneously more rapidly, most likely due to cerebral swelling associated with other injuries.23 Factors associated with deterioration requiring surgical intervention after initial conservative therapy have included temporal location, heterogeneous density on CT, initial CT done within 6 hours of injury, and significant primary brain injury with skull fracture, causing delayed EDH not seen on ultra-early CT21,42,43 Mortality in the surgical subset of EDH patients has been reported between 0% and 41%, and is lower in the pediatric group (~5%)10,11,13,28,44 Mortality has also decreased with the advent of CT scanning, development of trauma systems, and neurosurgical specialty intensive care units.3,7,25,45,46 When examined as a contributor to mortality from TBI by lesion type in a large multicenter study, EDH had a fairly low mortality index (percent mortality x percent incidence) compared with other lesion types.9 Functional outcome and mortality are affected by the following clinical findings: age, neurological status (coma or lucid interval, GCS motor score, focal neurological deficit, pupillary status), time to evacuation/decompression (for at least a subset of patients), intracranial pressure (ICP) elevations, and medical complications.10,11,20,27,28,47,59 Radiographic (CT) findings affecting outcome from EDH include: hematoma volume, degree of midline shift, compression of cisterns, associated intracranial lesions, signs of active bleeding (heterogeneous density), presence of a skull fracture, and fracture across a meningeal artery, vein, or dural sinus.12,24,20,27,29,48,62 Though several reports have correlated hematoma volume with outcome, at least one retrospective study found that hematoma volume did not correlate with either the preoperative neurological condition or the 6-month outcome.49 Correlation of physical findings and definitive therapy is, however, important, as demonstrated in two studies that determined that patients in whom the latency period between the onset of anisocoria and surgical evacuation was shorter had better outcomes and lower mortality than those for whom surgery was delayed47,50 and in two others that correlated outcome (including mortality) to time between onset of coma and evacuation.12,51 Others have found similar good overall outcomes after EDH without focal deficits,12,51 but decreasing rates of good outcome with, in order, hemiparesis, hemiparesis and anisocoria, decorticate posturing, decerebrate posturing, and fixed bilateral pupils occurring preoperatively.29 Children fare better than adults in general.19 Mortality and outcome are improved for patients transferred directly to a neurosurgical institution over those transferred in from outlying facilities (correlating with time to evacuation).3,26,52 While aSDH has been described in a myriad of clinical conditions (acquired coagulopathies, anticoagulation therapy, congenital bleeding disorders, arteriovenous malformations, aneurysm rupture, cancer, meningioma, cardiac surgery, spinal epidural catheter insertion, depth electrode use, cocaine use, near-drowning), the most common cause by far is trauma. Acute traumatic SDH is most often the result of MVA, but in older subpopulations, falls represent a higher proportion of etiologies. MVA is more often associated with a comatose state in aSDH patients, indicative of the high-velocity mechanism causing a greater degree of underlying brain trauma.53,54 Etiology of bleeding is from tearing of cortical surface arteries or veins or cerebral contusions, but often a bleeding source cannot be determined with certainty at operation.55,56 Acute bleeding into a pre-existing cSDH may also occur.56 Arterial sources may represent a particularly treacherous entity due to rapid expansion and cerebral compression and herniation. A large proportion of aSDH patients present in coma (GCS <9).7,14,57,58 Lucid intervals are sometimes seen, more frequently in isolated aSDH54 and in elderly patients.59 Pupil abnormalities are noted in 30% to 50% of patients.14 In infants with open sutures, the first signs of an aSDH may be distention of the fontanelles and/or separation of the sutures, or seizures.60 After the sutures fuse, the presenting signs and symptoms mimic those of adults: nausea/vomiting, headache, deteriorating consciousness and neurological status, pupillary dilation, and/or focal neurological deficit (hemiparesis/hemiplegia or abnormal motor posturing). Isolated aSDH is seen in a minority of patients. In contrast to EDHs, most aSDHs are associated with intraparenchymal hemorrhage(s), subarachnoid hemorrhage, skull fracture, EDH, in addition to extracranial trauma, such as facial fractures, vascular injuries, limb fracture, thoracic or abdominal trauma.14,36 CT scanning is the diagnostic modality of choice. Acute SDH appears as a hyperdense crescent-shaped extra-axial collection (Fig. 6-5). Magnetic resonance imaging (MRI) can sometimes demonstrate aSDH better in cases of thin collections. Acute blood appears hyperintense on T1 and hypointense on T261 (Fig. 6-6). However, MRI is usually not a feasible test to obtain in the setting of acute TBI with symptoms of aSDH, and, while MRI can detect some lesions not seen on CT that may aid in prognostication, surgical lesions are uniformly not missed on CT scanning.62,63 “Hyperacute” blood may also be seen in aSDH34,64 (Fig. 6-7). Surgical evacuation of aSDH is necessary for patients with significant mass effect, regardless of GCS. Significant mass effect may be defined as thickness of the hematoma >10 mm or midline shift >5 mm.14 Patients in whom there is less mass effect, but who experience a neurological deterioration such as a decrease in the GCS score by 2 or more points, loss of pupillary reactivity, or pupillary dilation, or who experience elevations of ICP above 20 mm Hg should also be taken to surgery for evacuation, if possible.14 Surgical evacuation of aSDH is done via craniotomy, usually a very generous one. This may be done with or without duraplasty and bone flap removal, depending upon the degree of underlying parenchymal injury and swelling. Burr hole drainage is ineffective and associated with higher mortality.59,65–67 In cases where CT scanning is not available, if burr hole exploration is used to detect the presence of extra-axial hematomas for subsequent evacuation via craniotomy in TBI patients with signs of transtentorial herniation or brainstem dysfunction, they should be placed first on the side corresponding to the neurological deficit in frontal, temporal, and parietal locations, followed by contralateral burr holes if negative, to maximize the chances of identifying the lesion(s).5 Surgical decision making may be affected by age, as aSDH tends to be better tolerated in the elderly patient with an atrophic brain; however, elderly patients with very low GCS scores, presentation in coma, and at least one abnormal pupil uniformly do poorly, with significant contributions to mortality from pre-existing conditions and multisystems failure.68,82 Very thin layers of aSDH, variously termed “sliver” or “smear” subdural hematomas, deserve special caution if accompanied by midline shift out of proportion to the size of the aSDH. The difference between the thickness of the aSDH and midline shift predicts outcome, with increasing disability and mortality when midline shift exceeds thickness by larger and larger intervals69 (Fig. 6-7). This phenomenon signals significant underlying brain injury and edema, and surgical evacuation of the clot is often helpful. Accompanying duraplasty and removal of the bone flap for delayed replacement may be necessary to accommodate significant hemispheric edema and prevent ongoing brainstem compression and elevated ICP. Intraoperative consideration must also be given to removal of underlying intraparenchymal hematomas or contusions, and much less commonly, partial lobectomy. Gentle elevation of the temporal lobe with relief of uncal herniation may be of some benefit. Figure 6-5 A large left hemispheric acute subdural hematoma is demonstrated on this CT scan (A) of the head, without contrast. Windowing the scan differently results in better demonstration of the lesion (B) Mass effect on the left hemisphere, left lateral ventricle, and cisterns are noted, with midline shift proportional to the thickness of the aSDH.
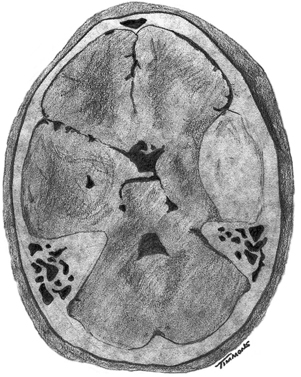
Epidural Hematoma
Epidemiology
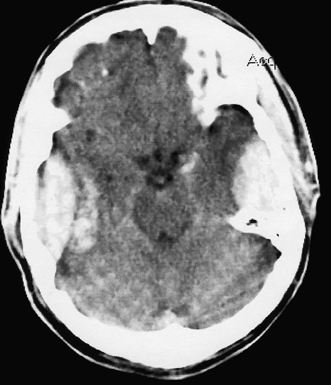
Pathogenesis
Diagnosis
Clinical Manifestations
Radiographic Findings
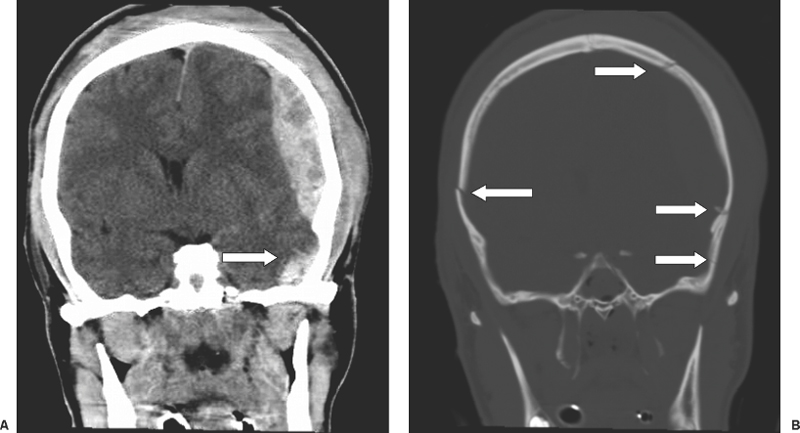
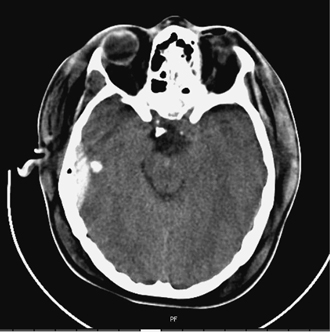
Treatment
Operative
Nonoperative
Outcomes
Acute Subdural Hematoma
Epidemiology
Pathogenesis
Diagnosis
Clinical Manifestations
Radiographic Findings
Treatment
Operative
Extra-Axial Hematomas
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








