(1)
Division of Critical Care Neurology, Mayo Clinic, Rochester, Minnesota, USA
Abstract
This chapter discusses the evolution of management of critically ill neurologic patients. This chapter discussed six crucial developments. Management of increased intracranial pressure, mechanical ventilation in neurologic patients, therapeutic hypothermia, and care of spinal cord injury are some of the topics discussed.
Short Historical Note 34
INCREASED INTRACRANIAL PRESSURE AND HYPERVENTILATION
The Title of the Paper
Gibbs FA, Gibbs EL, Lennox WG. Changes in human cerebral blood flow consequent on alterations in blood gases. Am J Physiol. 1935;111:557–63.
Fig. 5.1
Title page
The Paper and the Times
For quite some time, physiologists felt that cerebral blood flow was entirely dependent on systemic arterial blood pressure and passively followed any fluctuation in pressure. Even distinguished physiologists such as Sherrington, Bayliss, and Hill were unconvinced that a specific vasomotor regulation existed that was controlled by cerebral vasomotor nerves.
However, earlier studies, most prominently by Forbes and Wolff in 1928, found in experiments with cats that brain circulation was not regulated from a distance but that arteries constricted after direct application of epinephrine or stimulation of the cervical sympathetic nerves. The experiments also showed dilatation following stimulation of the vagus nerve [18].
There also was interest in whether cerebral blood flow could be altered by changes in blood gases [35]. Animal experiments showed that cerebral blood flow was dependent on carbon dioxide (CO2) or oxygen tension of blood and brain tissue and that cerebral blood flow could vary with the CO2 tension of arterial blood. Gibbs and Lennox from the Neurologic Unit of Boston City Hospital, the Department of Neurology of Harvard Medical School and Laboratory of Physiology of Yale University School of Medicine did one of the first experiments in healthy volunteers [20] (Fig. 5.1).
The Details of the Paper
The experiment involved normal volunteers who agreed to have a fine stylet introduced into the jugular vein. The tip was heated by means of a constant electrical current to a temperature that was slightly higher than the blood. The assumption was that if blood flowed faster past the tip, the tip would be become cooler; if the blood flow slowed down, the tip would become warmer. Volunteers were asked to breathe deeply and exhale forcibly for several minutes. This resulted in a sudden and pronounced decrease in blood flow through the internal jugular vein. Additionally, the CO2 content of the blood was increased from a tank containing a gas mixture of 10% CO2 and 90% oxygen that resulted in a marked increase in cerebral blood flow. Systemic arterial blood pressure remained largely constant, which then would allow the experiment to demonstrate that cerebral blood vessels change in caliber due to changes in concentration of these parameters. Increase of blood CO2 resulted in dilatation and decrease resulted in constriction of the arterioles of the brain. The effect of hyperventilation on a normal volunteer is shown in Fig. 5.2.
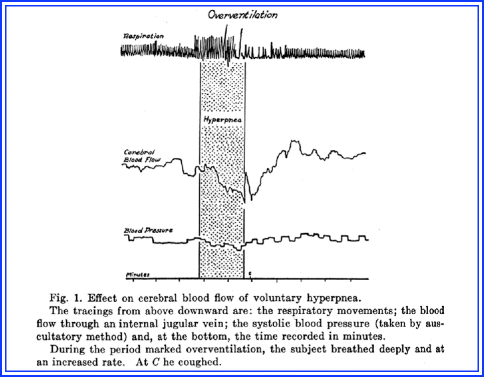

Fig. 5.2
The effects of hyperventilation
The authors concluded:
We find that “blowing off” CO2 from the alveoli of the lungs and from the blood caused a decrease in cerebral blood flow, whereas breathing a high concentration of CO2 caused an increase. The interesting question arises concerning the mechanism responsible for these changes in flow. Alterations in systemic arterial blood pressure may play a part, but lack of any close correlation of blood flow and blood pressure curves requires another explanation. The explanation lies, we believe, in alterations in caliber of the cerebral blood vessels in response to changes in the concentration of the blood gases. Increase of the blood CO2 results in a dilatation and decrease results in a constriction of the arterioles of the brain.
The Message and Acceptance
These experiments were followed by studies of situations where there was documented increase in intracranial pressure (ICP). Most noticeable was Lundberg’s paper that documented a reduction of increased ICP using hyperventilation [39] (Fig. 5.3). Lundberg’s paper reported on 18 patients with mostly glioblastomas, in whom hyperventilation markedly reduced ICP. Lundberg’s paper clearly defined the clinical practice of hyperventilation using the ventilator to manipulate blood CO2. Changes in respiratory minute volume induced rapid change in ventricular fluid pressure in the opposite direction. Other observations were made that showed that the flow of cerebrospinal fluid on open drainage was greater during spontaneous breathing than during hyperventilation.

Fig. 5.3
Lundberg’s monograph on hyperventilation
Over the years, the induction of respiratory alkalosis through hyperventilation has remained a rapid and fairly reliable method for controlling ICP during neurosurgical procedures and in the intensive care unit. As a corollary, these findings also demonstrated that CO2 retention could potentially result in increased ICP. More recently, evidence has been presented that hyperoxemia could further induce vasoconstriction in hyperventilated patients with traumatic brain injury [57].
Acute hyperventilation remains a very effective way of reducing ICP. The effects of hyperventilation on cerebral blood flow and ICP are not that significant after several hours and the prolonged use of hyperventilation has been criticized for its potential adverse effects [55]. In one prospective study, patients with severe traumatic brain injury (TBI) were randomized to prophylactic hyperventilation for 5 days with an average arterial PCO2 of 25 mmHg or to management without hyperventilation with an average arterial PCO2 goal of 35 mmHg. At 3 and 6 months, comatose patients in the hyperventilation group had significantly worse outcomes [48]. A recent study that monitored brain tissue oxygen found that even a small reduction in arterial PCO2 caused a decrease in cerebral oxygenation in comatose patients with TBI, but found that there was minimal cerebral vessel reactivity to arterial PCO2 changes several days from the insult. It appears that aggressive hyperventilation can cause cerebral blood flow to closely approach an ischemic threshold, and this may explain a potentially more harmful outcome [31].
The neurometabolic changes with acute hyperventilation in the setting of severe traumatic brain injuries have also been studied, but there are contradictory results and comparatively few patients have been reported [9, 13]. When measured, both brain tissue CO2 and the jugular vein oxygenation are reduced with hyperventilation. Other studies have found that moderate hyperventilation may significantly increase accumulation of lactate.
Short Historical Note 35
INCREASED INTRACRANIAL PRESSURE AND OSMOTIC AGENTS
The Title of the Paper
Faye T. Administration of hypertonic salt solution for relief of intracranial pressure. JAMA. 1923;80:1445–8.
Haden RL. Therapeutic applications of the alteration of brain volume. JAMA. 1919;73:983–4.

Fig. 5.4
Title page

Fig. 5.5
Title page
The Paper and the Times
Reducing the intracranial volume of the brain by extracting water was of paramount interest to neurosurgeons. Relief of increased intracranial pressure (ICP) in patients with a mass and headache and vomiting resulted in improvement of symptoms before surgery and also could reduce swelling seen during surgery.
Neurosurgeons Lewis Weed and Paul McKibben were among the first to study the effects of IV salt solutions on cerebrospinal fluid (CSF) pressure in cats in their Army Neurosurgery Laboratory at Johns Hopkins Medical School. They found that hypertonic solutions lowered the CSF pressure—and vice versa—hypotonic solutions increased CSF pressure [74]. These hypertonic solutions were 30% sodium chloride or saturated sodium bicarbonate. They compared its effect to Ringer’s solution (NaCl 0.9%, KCL 0.04%, CaCl 0.025%) and to distilled water. In some cats, trephine openings were added to observe the changes after administration of a hypertonic or hypotonic solution—a shrunken or swollen brain. No changes were seen with Ringer’s solution. The investigators could only conclude that changes in the osmotic value of blood were responsible for changes in brain tissue. The investigators surmised that CSF pressure reflected intracranial volume.
Cushing and Foley also tested 2% sodium chloride solution introduced into the duodenum or rectum of a cat. They could document significant reduction of CSF pressure [12]. They found little change in blood pressure and found no changes in pulse or respiration. Cushing and Foley, to use IV injection of 35% sodium chloride solution during neurosurgical procedures [12] and to use it in patients with brain tumor and “cerebral hernias” before a decompression operation. Using hypertonic saline by mouth could result in “occasionally striking results … in which case the tense convex protrusion became a soft concave area over the decompression site” [12].
The simple physics of salt and sugar were applied in the early 1920s, and many physicians became familiar with the fact that both concentrated salt and glucose solutions could create an osmotic effect that would draw water out of the brain [29, 64]. Hypotonic solutions would have the opposite effect, and isotonic solutions had no such effect.
The Details of the Paper
Faye initially used magnesium sulfate by rectum and demonstrated its usefulness—a marked decrease in ICP in 16 patients (Fig. 5.4). The authors used magnesium sulfate because they found that sodium chloride solution by mouth often was associated with vomiting and significant distress such as thirst and gastritis. Additionally, they reported a series of 15 patients undergoing surgery for brain tumor who were treated with IV hypertonic sodium chloride solution and found marked reduction of pressure using a variety of salt solutions varying from 15% to 35%. They also documented that the most marked dehydrating effects were seen in patients with hydrocephalus and recommended its use in exploratory craniotomies.
Haden used 25% concentrated glucose to increase the osmotic value in blood and cause a diuretic effect (Fig. 5.5):
The one medical condition in which we are most interested in intracranial pressure is cerebrospinal meningitis. The marked increase in pressure in this disease is certainly one of its most serious aspects. There is an increase both in the amount of spinal fluid and in the direct volume of the brain. The increased pressure is often the factor determining the outcome of the disease [29].
The patient was a 21-year-old man with “epidemic cerebrospinal meningitis.” When he deteriorated and became comatose, a marked improvement was noted after 200 mL of 25% IV glucose solution and even more the next day when an additional dose of 550 mL was administered. Another case of “pulmonary consolidation, high fever and stupor” was described as “measles toxemia with cerebral edema,” but without much details other than a marked improvement in responsiveness. Haden suggested to use the glucose infusion slowly—1 h—and to repeat it in 12 h.
The Message and Acceptance
At the time, salt solutions were commonly used in operative settings. Morrissey—after Fay’s example—published several cases of improvement of CSF pressure using oral magnesium sulfate crystals dissolved in water but was not impressed by its pressure-lowering effect. Morrisey confirmed in his experiments that IV hypertonic saline was quite effective [46].
However, in the 1960s, urea and glycerol were on the radar [8, 32, 80]. Glycerol increased plasma concentration, and therefore its effect would be to draw liquids from the tissues. Glycerol was found to promote diuresis, but it became apparent that the drug effects in nephrectomized animals did not differ from those in animals with normal kidney junction.
Cantore and associates from the Neurological Institute of Rome University in Italy reported on animal experiments and patients and successfully used oral glycerol for the reduction of ICP [10]. Few clinical patient data were provided, but in the scheme of things, it became just another important observation that would push the use of glycerol in neurosurgical practices. Oral glycerol lacked the toxic effects of intravenously applied glycerol.
And so, the salt versus sugar dilemma started. Mannitol replaced glycerol and hypertonic saline eventually may replace mannitol [14, 67, 73, 77]. Mannitol continues to be the first line of treatment and is most favored because of ease of use—rapid administration without the need of central access—and a substantial clinical experience. There is an unsubstantialed concern that excessive administration of mannitol may be harmful as a result of accumulation in brain parenchyma and potentially worsening edema due to reverse osmotic shift.
Hypertonic saline is now more commonly used for treatment of increased ICP [45, 53]. The osmolality of hypertonic saline in the higher concentrations is several folds higher than mannitol and therefore may draw more water. Hypertonic solutions shift fluid from the intracellular space to the extracellular space, and there is little intravascular expansion. Prospective comparisons between hypertonic solutions may be helpful. Concerns about adverse effects are real and involve volume overload, phlebitis at the infusion site, and hyperchloremic acidosis [53].
Short Historical Note 36
CARE OF THE COMATOSE PATIENT
The Title of the Paper
Eckenhoff JE. The care of the unconscious patient. JAMA. 1963;186:541–3.

Fig. 5.6
Title page
The Paper and the Times
Treatment of coma—as it is now—was disease specific and took quite some time to evolve. Most physicians understood the need to treat increased intracranial pressure (ICP) after trauma. Around the turn of the century, physicians confronted with coma after traumatic brain injury would consider trepanation to prevent “mortal danger.” Marburg—a neurologist from Vienna—mentioned that the two most important issues to consider in comatose patients were increased intracranial pressure and regulation of circulation and respiration. Drugs to be used in hypotension included atropine, strychnine, ergotin, ephedrine, and caffeine [41].
In the second half of the twentieth century, publications were still mostly concerned with management of specific systemic disorders causing coma, often diabetes [16, 37]. Specific treatment of diabetic, hepatic, and thyrotoxic coma or other endocrine crisis dominated the literature [34, 59, 66].
In the neurologic literature and textbooks, very little was written on care of the comatose patient. Depending on the severity of coma, these patients were mostly under the care of anesthesiologists or internists. Neurologists would consult and assess the cause and chances of recovery. Before neurologists Fred Plum and Jerry Posner would write their original textbook on the diagnosis of coma and stupor—as the title implied with less focus on care—other specialists authored papers on management. One paper concerned itself with the nursing care of the comatose patient, but a systematic approach to the comatose patient was only briefly mentioned in textbooks without sufficient attention to detail. Some review articles mentioned use of oxygen, water depletion, and nursing position [3, 43, 44, 58, 47, 78]. Care at the time was mostly directed toward general patient care and avoidance of hypotension and hypoxemia—the precursors of the ABC of resuscitation.
The Details of the Paper
Anesthesiologist Eckenhoff published one of the first comprehensive papers on care of the comatose patient in 1963 (Fig. 5.6). This review was read before the section on Nervous and Mental Diseases during the 112th annual meeting of the American Medical Association. The major theme was induced hypothermia, airway, and pulmonary care. Eckenhoff emphasized the disappearance of normal respiratory protective mechanisms in coma. “In sleeping man, the protective respiratory reflexes are active. Olfactory nerves warn of offending odors and tracheal reflexes react to the presence of foreign material. Respiratory cilia beat secretions toward the bronchi and trachea whence their presence precipitates coughing.”… “It is only when alcohol, drugs or disease block the reception of the danger signals….” The paper discussed airway management (lateral position placement, endotracheal tube, or tracheotomy) and adequate oxygenation, but also warned for a rise in arterial carbon dioxide. Eckenhoff remarked:
It is probably wise to increase the inspired O2 content of all comatose patients, but the need for supplemental oxygen can only be determined accurately by analysis of the arterial blood. If the PO2 is below 90 mm Hg assuming a normal hemoglobin content, then the concentration of inhaled oxygen should be increased. A properly placed nasopharyngeal catheter with oxygen flowing at six liters per minute will increase inspired oxygen concentration to 35%. The highest oxygen concentration likely with an oxygen tent is 50% with careful application. Oxygen hoods or masks offer higher concentrations, but these are seldom needed.
Comments were made on the tracheostomy timing (“there should be no urgency in doing this procedure”) and benefits due to reducing respiratory dead space and increasing alveoli ventilation, easy removal of respiratory secretions, and improved mechanical ventilation. The review mentioned a diminished sympathetic tone and predisposition to severe hypotension while turning the patient. A specific note was made of temperature management:
Brain damage after trauma can be minimized by lowering body temperature to 86 to 88 F (30.0 to 31.1 degrees of Celsius) for several days after the injury. While this is now a common technique in the treatment of trauma and cerebral aneurysm, it is only beginning to receive trial in the treatment of stroke per se. Theoretically, it could be of value and deserves further exploration.
Accumulation of carbon dioxide and its consequences—increase in cerebrospinal fluid pressure and increase in catecholamines—resulting in peripheral vasostriction, hypertension, increase in brain volume, and cerebral edema was also mentioned.
The Message and Acceptance
Another major work appeared as a chapter and involved more specifics. Rosomoff and Safar’s chapter clearly spells out most of the concerns and elaborates on management of fever and the benefits of cooling [63]. Most attention was directed to airway and pulmonary care, but recommendations on fluid management included use of 5% in Ringer’s solution or 5% glucose in 0.2% saline in patients with increased ICP. However, Rosomoff and Safar warned that IV solution without solutes could increase ICP and suggested approximately 2,500 mL of fluids.
In a remarkable statement on generalized convulsions and antiepileptic drugs, the author warned against the use of phenobarbital, and other convulsants as acute management interventions. “Suppression of the cortical electrical hyperactivity does not seem to be essential for emergency management, so long as the respiratory manifestations are controlled.”
The paper was influential in further elaborating on the treatment of hypothermia. Cooling was achieved with wrapping patients in a cooling mattress and using a “shivering cocktail” (meperidine, promethazine, and phenobarbital).
Why it took so long to organize and teach the management of comatose patients remains unclear. A simple, although inadequate, answer is that neurology was a more a “diagnostic specialty”. Care of the comatose patient became more standardized only in the third edition of Plum and Posner’s text on the diagnosis of stupor and coma. They suggested that the treatment should involve the following steps: adequate oxygenation, maintenance of circulation, glucose administration if strong suspicion of hypoglycemia, reduction of ICP, treatment of seizures, treatment of any infection, correction of acid–base balance, normalization of body temperature, administration of thiamine, consideration of antidotes, control of agitation, and protection of the eyes.
Short Historical Note 37
MECHANICAL VENTILATION IN NEUROLOGIC DISEASE
The Title of the Paper
Lassen HC. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet. 1953;1:37–41.
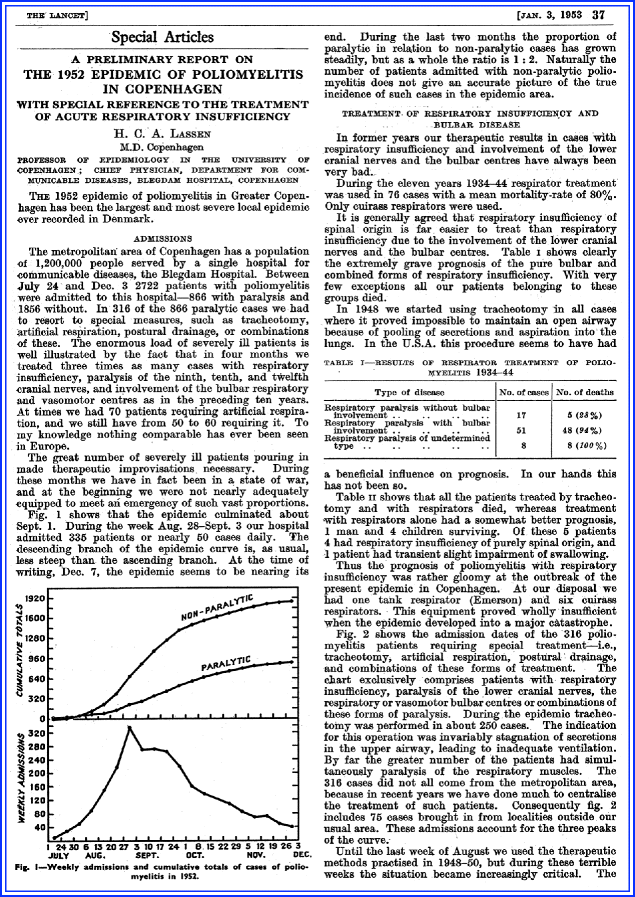
Fig. 5.7
Title page
The Paper and the Times
The history of ventilation goes back many centuries with experiments by Galen, Paracelsus, Vesalius, and Harvey. According to Westhorpe and Ball, the first description of airway management was by Arab physician Ibn Sina, also known as Canon of Medicine, and was published in the first half of the eleventh century [75]. “When necessary a cannula of gold or silver or other suitable material is advanced down the throat to support inspiration” [15].
Interestingly, modern mechanical ventilation started with a devastating neurologic disease. Mechanical ventilation has its origins in the early 1800s with negative pressure ventilation and tank respirators. The Drinker–Shaw and the Emerson “iron lungs” are best known. Poliomyelitis was very much on Philip Drinker’s mind when he worked on his device:
A number of patients have come under our observation who have shown intercostal paralysis and considerable weakness of the diaphragm early in the paralytic stage of anterior poliomyelitis; yet the accessory muscles and the diaphragm have been able to support sufficient respiratory movements to keep the patient alive until, in the course of a few days, the paralyzed intercostal muscles began to improve and the patient went on to almost complete recovery [15].
He would invent a machine that incorporated electrically driven blowers and create inspiration with negative pressures and expiration with positive pressures. Within the chamber—sealing the patient at the neck—a negative pressure caused the abdomen and thorax to expand with air flowing in. A cycle was produced by returning to atmospheric pressure or after some positive pressure recoil of the chest and abdomen occurred. Although they maintained support in patients in an often decrepitude state—and many could be liberated from the device—the long term problems with these monstrous devices were well known and included technical difficulties and inability to secure the airway.
In the 1950s, these negative pressure ventilators were the only available ventilators and its dramatic shortage during the zenith of the polio epidemic necessitated other ways of ventilatory support. Positive pressure ventilation (PPV) and tracheostomy became commonplace after the poliomyelitis epidemics.
The most notorious epidemic was in 1952 in Copenhagen [2, 75]. Between July and December, the Blegdam Hospital received 2,722 patients with poliomyelitis; 316 had respiratory insufficiency of varying degree. Most medical historians consider this event and the emergence of respiratory care units soon thereafter the beginnings of critical care (Chapter 2).
The Details of the Paper
The paper vividly describes that the hospital was overwhelmed with only one iron lung and six cuirass respirators available (Fig. 5.7). Lassen said the hospital was in “a state of war.” The report describes the use of tracheostomy and manual ventilation, and with this approach the care of patients became much more organized. The new approach included early tracheostomy just below the larynx, suctioning and bronchoscopy via the tracheostomy, aggressive postural drainage, and PPV via a cuffed tube. A tracheostomy was performed with excision of a square opening in the anterior wall of trachea, and the widest possible rubber tube with inflatable cuff was inserted. Repeat suction of the trachea and postural drainage and bag ventilation with insufflation of a mixture of 50% oxygen and 50% nitrogen was next.
This equipment was used in over 150 patients, but initial mortality rates remained high, reaching 80%. Of 55 men treated with tracheostomy and bag ventilation, 31 died; of 43 women, 17 died; and of 74 children, 29 died. The paper stated, “During the several weeks we had 40–70 patients in our hospital requiring continuous or intermittent bag ventilation. To do this we have employed about 200 medical students daily.”
There were enormous challenges to manage a large number of patients with poliomyelitis at the same time (75 patients under manual ventilation requiring next to the medical students, 260 nurses to provide care, and 27 technicians to control the machinery). Early tracheostomy with bag ventilation and repeated suctioning and positioning could bring mortality down from 80% to 40%. The anesthesiologists also recognized that with PPV, pulmonary edema could occur. The patient, therefore, was kept relatively dry, and there was specific attention to the presence of a “too high” pressure during ventilation that was recognized as inciting pulmonary edema.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








