Gastric and Genitourinary Function and the Brain
Alden Doerner Rinaldi
Charles C. Esenwa
INTRODUCTION
The brain shares intricate connections with the gastrointestinal (GI) and genitourinary (GU) organ systems. Injury to the nervous system at any anatomic level and by a number of mechanisms—ischemia, trauma, inflammation, infection, toxic-metabolic insult, developmental anomaly, or degeneration—can provoke GI or GU symptoms. Inversely, primary GI disorders may have deleterious effects on neurologic function. In both cases, the human consequences, in terms of quality of life, morbidity, and mortality can be enormous. Here we describe (1) the general neurologic mechanisms that lead to disruption of the healthy physiologic function of the GI and GU systems, (2) specific neurologic disorders that have comorbid GI or GU manifestations, and (3) GI disorders that are associated with neurologic complications.
NEUROANATOMY
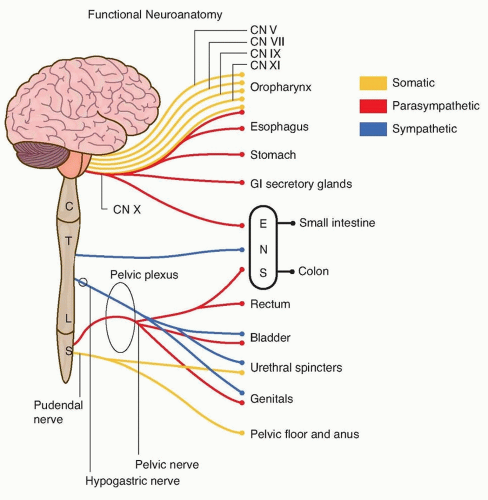
The autonomic nervous system is central in the regulation of the GI and GU systems. Autonomic impulses are mediated by the cortex and hypothalamus and affect nuclei in the brain stem, thoracolumbar, and sacral spinal cord. Parasympathetic cholinergic outflow from the dorsal nucleus of the vagus nerve in the medulla promotes the vegetative functions of secretion, digestion, absorption, and gut motility, whereas sacral parasympathetics stimulate voiding, defecation, and tumescence.
Sympathetic adrenergic outflow from the thoracolumbar cord directly opposes parasympathetic function by decreasing secretions, gut motility, and splanchnic blood flow while also increasing sphincter tone to promote urinary and fecal continence. Somatic motor neurons arising from Onuf’s nucleus at the S2-S4 segments of the sacral cord pass via the pudendal nerve to innervate muscles of the pelvic floor and sphincters at the anus and urethra maintaining volitional control over continence (Fig. 121.1).
GASTROINTESTINAL MANIFESTATIONS OF NEUROLOGIC DISEASE
The enteric nervous system (ENS) is a quasi-independent branch of the autonomic nervous system with an estimated 400,000 to 600,000 neurons, nearly equal to that of the entire spinal cord.
Its function is to (1) orchestrate gut motility, (2) control secretomotor function, (3) regulate local blood flow, (4) control fluid and nutrient absorption, and finally, (5) interact with and modulate the enteric immune system. It has two anatomically distinct subdivisions. The submucosal plexus, which includes Meissner plexus, is located in the gut wall and monitors the local epithelial environment exerting secretomotor control in response to parasympathetic stimuli, whereas the myenteric, or Auerbach, plexus located superficially in the muscular layer of the gut, mediates motility through both sympathetic and parasympathetic inputs. The most proximal and distal parts of the GI tract are somatically innervated, making these areas particularly susceptible to neurologic injury.
Its function is to (1) orchestrate gut motility, (2) control secretomotor function, (3) regulate local blood flow, (4) control fluid and nutrient absorption, and finally, (5) interact with and modulate the enteric immune system. It has two anatomically distinct subdivisions. The submucosal plexus, which includes Meissner plexus, is located in the gut wall and monitors the local epithelial environment exerting secretomotor control in response to parasympathetic stimuli, whereas the myenteric, or Auerbach, plexus located superficially in the muscular layer of the gut, mediates motility through both sympathetic and parasympathetic inputs. The most proximal and distal parts of the GI tract are somatically innervated, making these areas particularly susceptible to neurologic injury.
DYSPHAGIA
The process of deglutition is initiated in the cerebral cortex and overseen by the nucleus ambiguus in the medulla. Chewing, bolusing, and initiation of the swallow reflex occur in the oropharynx through a coordinated effort involving multiple cranial nerves. The swallow reflex and esophageal peristalsis are the involuntary phases and are overseen by glossopharyngeal and vagal inputs. Difficulty with swallowing, or dysphagia, can manifest as drooling, inability to effectively maneuver the food bolus, a sensation of esophageal fullness, and choking or coughing when attempting to ingest food.
A disease process affecting the primary motor cortex or supplementary motor areas, such as stroke or neurodegenerative dementia, can disrupt the preparatory and oral phases of swallowing. Direct injury to the brain stem or corticobulbar tracts by ischemia, the Parkinson-plus syndromes, or multiple sclerosis (MS) can disrupt inputs to the nucleus ambiguus, affecting the involuntary phase of swallowing. Motor neuron disorders such as amyotrophic lateral sclerosis (Lou Gehrig disease) or spinal and bulbar muscular atrophy (Kennedy disease) cause prominent dysphagia by affecting the motor neurons that supply the muscles of mastication, the oropharynx, and upper esophagus. Peripherally, disorders of the neuromuscular junction, such as myasthenia gravis and botulism, and disorders of muscle, including the muscular dystrophies and inflammatory myopathies, can also have significant effects on all phases of deglutition.
Videofluoroscopy has been the gold standard in the evaluation of dysphagia and direct visualization using videoendoscopy is now being used more commonly. In stroke, where deglutition is expected to recover, percutaneous endoscopic gastrostomy (PEG) is sometimes used as a bridge to independent swallowing. Dysphagia due to a degenerative disorder, although mitigated by swallow therapy, will naturally progress to malnutrition, dehydration, and ultimately carries a high risk of aspiration pneumonia.
ESOPHAGEAL DYSMOTILITY
The lower esophageal sphincter (LES) is controlled by the dorsal motor nucleus of the vagus nerve and is meant to serve as a oneway valve for food contents passing from the esophagus to the stomach. Achalasia is characterized by loss of esophageal peristalsis with sustained contraction of LES leading to megaesophagus and a classic “bird’s beak” appearance on videofluoroscopy. Although typically a primary idiopathic disorder, there is evidence implicating an underlying inflammatory or autoimmune process affecting the ganglion cells of the myenteric plexus. Similarly, in the chronic stages of Chagas disease, achalasia can occur by infectious destruction of the myenteric plexus by the protozoa Trypanosoma cruzi
GASTROPARESIS
Peristalsis in the stomach is controlled by independent rhythmic contractile waves paced by the interstitial cells of Cajal and overseen by vagal inputs. Gastroparesis is delayed gastric emptying from impaired antral motility and usually presents as postprandial bloating, pain, nausea, and vomiting. Diabetic vagal neuropathy and diabetic autonomic neuropathy are common causes that can similarly affect bowel motility, causing alternating diarrhea and constipation. Prokinetic agents such as metoclopramide, cisapride, and erythromycin are used to treat acute exacerbations, whereas severe cases may require venting gastrostomy with feeding enterostomy.
STRESS GASTRIC ULCER
Neurologic dysfunction from traumatic brain injury, spinal cord injury, or neurologic surgery predisposes to stress-related mucosal injury and hemorrhage. Stress ulcers are particularly exacerbated by injury involving brain stem and diencephalon. Dysregulation of hypothalamic function with simultaneous overactivation of parasympathetic and sympathetic efferents both increase gastric acid production and compromise mucosal protection. Stress ulcer prophylaxis is recommended in patients with coagulopathy or those requiring mechanical ventilation but should also be considered in patients with traumatic brain or spinal cord injury. Proton pump inhibitors serve as first-line therapy.
BOWEL DYSMOTILITY
Motility of the small and large intestines is coordinated exclusively by reflex loops made up of motor neurons, interneurons, and intrinsic sensory neurons. Primary Hirschsprung disease is a condition in which this neuronal network fails to form. Failure of neural crest cells to migrate during development leaves a section of colon or rectum without functional enteric plexus. Newborns can have delay in passage of meconium and in severe instances will have massive secondary dilation of the bowel termed megacolon. In less severe cases, diagnosis can be delayed to late childhood and even adulthood. Patients in this subgroup come to medical attention because of chronic symptoms of constipation. Diagnosis is suggested by videofluoroscopy and is made by rectal biopsy. Surgical resection of the affected bowel is the mainstay in treatment. Destruction of the enteric plexus by T. cruzi infection in Chagas disease is a secondary form of megacolon that has a similar diagnostic and therapeutic approach.
INCONTINENCE
Much like the esophagus, autonomic and somatic nerves innervate the anorectum. Reflex muscle contractions and paired inhibitions drive fecal movements through the rectum and into the anal vault. Motor neurons arising from Onuf’s nucleus at the sacral level traverse the lower sacral plexus and pudendal nerves, ultimately exerting their effect on the external anal sphincter and muscles of the pelvic floor. Lower motor neuron injuries at this level cause sphincter dysfunction with loss of formed stool and fecal incontinence. Anorectal manometry and anal sphincter electromyography can help in such cases.
URINARY MANIFESTATIONS OF NEUROLOGIC DISEASE
The lower urinary tract (LUT) serves as both reservoir and outlet by the reciprocal action of detrusor and urethral sphincter muscles.
Anatomically, the periaqueductal gray (PAG), located in the pons, integrates rostral input from the midbrain, limbic system, and cortex to promote bladder compliance and storage through a direct tonic inhibition of the pontine micturition center (PMC). This inhibition results in a storage reflex by which suppression of parasympathetic and activation of sympathetic pathways causes synergistic detrusor relaxation and urethral smooth muscle contraction to close the urethral outlet. A voiding reflex is similarly directed by the PAG, whereby release of the PMC activates parasympathetically driven circuits throughout the thoracosacral spinal cord. Cholinergic stimulation contracts the bladder, whereas nitric oxide (NO) released from nonadrenergic noncholinergic parasympathetic (NANC) fibers relaxes the internal urethral sphincter. This action is facilitated by volitional control of somatically innervated striated muscle of the external urethral sphincter to open the urethral outlet and permit micturition. These distinct reflexes may be disrupted by neurologic injury leading to symptoms that are broadly classifiable as (1) voiding dysfunction with hesitancy, intermittency, slow stream, straining, terminal dribble, and retention or (2) storage dysfunction with urgency, frequency, nocturia, incontinence, and altered bladder sensation (Fig. 121.2).
Anatomically, the periaqueductal gray (PAG), located in the pons, integrates rostral input from the midbrain, limbic system, and cortex to promote bladder compliance and storage through a direct tonic inhibition of the pontine micturition center (PMC). This inhibition results in a storage reflex by which suppression of parasympathetic and activation of sympathetic pathways causes synergistic detrusor relaxation and urethral smooth muscle contraction to close the urethral outlet. A voiding reflex is similarly directed by the PAG, whereby release of the PMC activates parasympathetically driven circuits throughout the thoracosacral spinal cord. Cholinergic stimulation contracts the bladder, whereas nitric oxide (NO) released from nonadrenergic noncholinergic parasympathetic (NANC) fibers relaxes the internal urethral sphincter. This action is facilitated by volitional control of somatically innervated striated muscle of the external urethral sphincter to open the urethral outlet and permit micturition. These distinct reflexes may be disrupted by neurologic injury leading to symptoms that are broadly classifiable as (1) voiding dysfunction with hesitancy, intermittency, slow stream, straining, terminal dribble, and retention or (2) storage dysfunction with urgency, frequency, nocturia, incontinence, and altered bladder sensation (Fig. 121.2).
VOIDING DYSFUNCTION
Lower motor neuron lesions of the sacral roots, plexus, or peripheral nerves cause parasympathetic denervation of the detrusor muscle resulting in a large, areflexic, and underactive bladder. When occurring alone, functional voiding symptoms may be difficult to differentiate from urinary outlet obstruction, but decreased rectal tone and impaired defecation often accompany. In the extreme form, the patient will have acute urinary retention, which is a urologic emergency. Causes include spinal cord shock from traumatic injury, Guillain-Barré syndrome, conus medullaris syndrome, and cauda equina syndrome; however, additional neurologic symptoms such as sensory loss, weakness, and areflexia will typically predominate in all of these. Less commonly, acute infection of the sacral roots by varicella or herpesviruses can also cause urinary retention accompanied by unilateral sacral pain, sensory loss, and classic zoster or herpetic rash. Opiates, anticholinergic medications, α-adrenoreceptor agonists, benzodiazepines, nonsteroidal anti-inflammatory agents, and calcium channel blockers can all cause reversible voiding dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree