Chapter 122 Heart Failure
Abstract
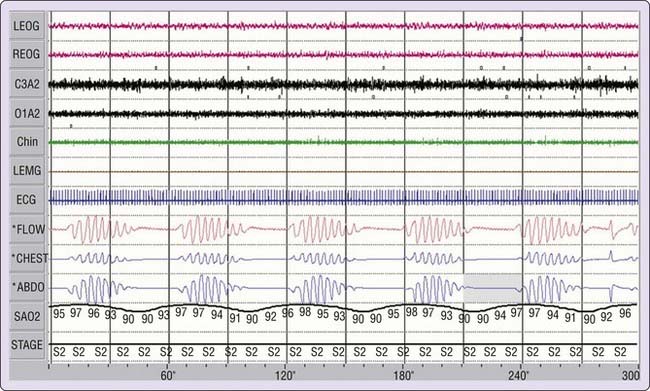
Heart failure has been known for more than 2 centuries to be associated with abnormal breathing patterns, and John Cheyne and William Stokes have been credited for its description—hence the eponym Cheyne-Stokes breathing.1,2 However, 37 years earlier, John Hunter,3,4 a British physician, was the first to describe this breathing pattern, which is characterized by gradual crescendo–decrescendo changes in tidal volume, commonly with an intervening central apnea (Fig. 122-1).5–9 We therefore refer to this pattern as Hunter-Cheyne-Stokes breathing. Periodic breathing is a pattern of breathing characterized by cyclic fluctuations in the amplitude of tidal volume.10 It consists of recurring cycles of apnea or hypopnea, or both, followed by hyperpnea. The apneas and hypopneas may be obstructive (i.e., the result of upper airway occlusion) or central.10 Obstructive sleep apnea–hypopnea is the most common form of periodic breathing in persons without heart failure. However, in patients with heart failure, both obstructive and central periodic breathing occur, although central sleep apnea–hypopnea is predominant.
Hunter-Cheyne-Stokes breathing (HCSB) is a form of periodic breathing with central sleep apnea that occurs in patients with systolic heart failure and has a long cycle time.11 The latter is an important feature of HCSB breathing and reflects the prolonged circulation time that is a pathologic feature of systolic heart failure. HCSB is a subjective description and is not readily quantifiable. For these reasons, the term central sleep apnea is preferable, and it also avoids misrepresentation, as credit for the discovery of breathing pattern has not been given to the original discoverer.
Central sleep apnea observed in awake patients with heart failure has been considered a rare entity and potentially an indicator of a terminal prognosis. However, like obstructive apnea, central apnea occurs primarily during sleep, and polysomnographic studies have reported a high prevalence of this disorder in ambulatory patients with stable heart failure.8,9,12–14
Epidemiology of Heart Failure and Sleep-Related Breathing Disorders
Heart Failure
Heart failure is approaching epidemic proportions and has become a major public health problem.15 It is estimated that it may contribute directly or indirectly to 266,400 deaths each year. The death rate increases progressively with advanced symptomatology, approaching 30% to 40% annually in patients with heart failure in New York Heart Association class IV. It is the largest single Medicare expenditure because it is the leading cause of hospitalization for patients older than age 65 years. Not surprisingly, therefore, the economic impact of heart failure is also huge, with an estimated cost of $29 billion in 2004 and $37 billion in 2009 (see Chapter 116, Table 116-1).15
It is estimated that 1.5% to 2% of the U.S. population has heart failure.15 Heart failure is a disorder of elderly persons, and its prevalence increases to approximately 6% to 10% in those older than 65 years. Furthermore, it is estimated that 20 million people may have asymptomatic cardiac dysfunction, and with time, these persons are likely to become symptomatic. Because of the increased average life span and improved therapy for ischemic coronary artery disease and hypertension, which are risk factors for heart failure, it is predicted that incidence and prevalence of heart failure will continue to rise in the 21st century.
Sleep Apnea in Systolic Heart Failure
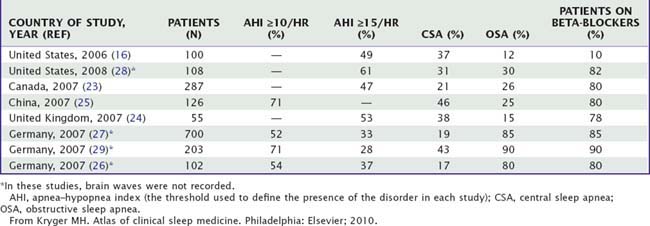
The prevalence of sleep-related breathing disorders has been systematically studied in patients with systolic heart failure.5 Polysomnographic studies6–14,16–25 and studies using respiratory channels26–29 show a high prevalence of sleep apnea in this population. Most recent large studies of consecutive patients with systolic heart failure are depicted in Table 122-1.
High prevalence rates have been reported in patients who have systolic heart failure and are awaiting transplantation,17 those with valve heart disease,18 and those with an implanted cardiac defibrillator.19,20
The most systematic prospective study of systolic heart failure16 involved 100 ambulatory male patients with stable, treated heart failure. Using an apnea-hypopnea index (AHI) of 15 events per hour or greater as the threshold, 49 patients (49% of all patients) had moderate to severe sleep apnea–hypopnea, with an average AHI of 44. In comparison, a population study of subjects without heart failure30 showed that 9% of working men and women aged 30 to 60 years had an AHI of greater than 15. An AHI of 5 or greater has been used to define the presence of a significant number of disordered-breathing events in obstructive sleep apnea–hypopnea syndrome.23 Therefore, with a much higher prevalence of sleep apnea observed in patients with heart failure than in the general population, systolic heart failure should be the leading risk factor for sleep apnea in the general population. In our studies,9,16 about 10% of the patients were on beta-blockers; however, recent studies continue to show a high prevalence of sleep apnea, both central and obstructive, despite the use of beta-blockers. Table 122-1 shows the prevalences of central and obstructive sleep apnea in the largest recent studies.16,23–29 Combining the results of these recent series, which used an AHI of 15 per hour of sleep as the threshold, 52% of 1250 patients with systolic heart failure have moderate to severe sleep apnea, 31% have central sleep apnea (CSA), and 21% have obstructive sleep apnea (OSA) (Fig. 122-2). However, there has been considerable variation in the prevalence of these two forms of sleep apnea in patients with systolic heart failure (see Table 122-1) which depends on a number of issues. The major reasons are the criteria used to define hypopnea, the accuracy of classification of disordered-breathing events (obstructive versus central, particularly in regard to hypopneas), the criteria used to define predominant obstructive versus central sleep apnea, the number of obese patients with heart failure enrolled, the level of arterial PCO2, and the severity of left ventricular systolic dysfunction.
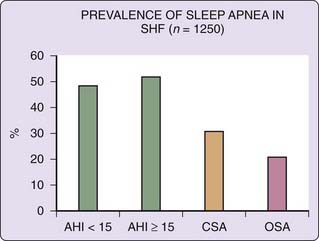
Figure 122-2 Prevalence of sleep apnea in systolic heart failure (SHF). The data presented combine a series of world sleep studies (see Table 122-1). AHI, apnea–hypopnea index; CSA, central sleep apnea; OSA, obstructive sleep apnea.
(Reproduced from Kryger MH. Atlas of clinical sleep medicine. Philadelphia: Elsevier; 2010.)
Sleep Apnea in Isolated Diastolic Heart Failure
Isolated left ventricular diastolic dysfunction (LVDD) with relative preservation of left ventricular systolic function is the most common form of heart failure in elderly patients. The pathophysiologic consequence of LVDD relates to a hypertrophied, noncompliant left ventricle shifting the pressure volume curve upward and to the left. Therefore, for a given left ventricular (LV) volume, LV end-diastolic pressure increases, resulting in elevated left atrial and pulmonary capillary pressures, pulmonary congestion, and edema. Hemodynamic studies show that pulmonary capillary pressure increases during the course of obstructive apnea, indicating the development of LVDD (see Chapter 120). Chronic repetitive exposure to negative swings in intrathoracic pressure, cyclic nocturnal hypertension and hypoxemia, and diurnal systemic hypertension could eventually result in LV hypertrophy and LVDD and failure.
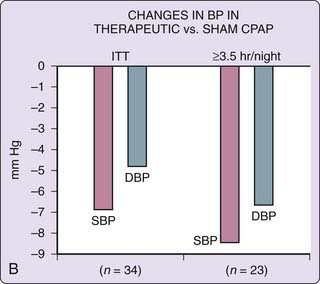
Studies30–32 suggest that OSA is associated with an increase in LV mass and that OSA-related cardiac structural changes may resolve with continuous positive airway pressure (CPAP) treatment. In the largest study,32 consisting of 2058 Sleep Heart Health Study participants, LV mass was associated with both apnea–hypopnea and hypoxemia indices after adjustment for age, sex, ethnicity, body mass index, smoking, systolic blood pressure, antihypertensive medication use, diabetes mellitus, prevalent myocardial infarction, and alcohol consumption. Furthermore, studies31,33 suggest that treatment with CPAP results in reversal of diastolic dysfunction.33 This has been confirmed by a randomized placebo (sham CPAP)-controlled trial, showing that after 12 weeks on effective CPAP therapy, there was a significant increase in the early-to-atrial filling velocity (E/A) ratio and a significant decrease in isovolemic relaxation and mitral deceleration time.
Overall, little is known about the prevalence of sleep-related breathing disorders and their impact in patients with isolated diastolic heart failure.34,35 Yet both disorders are prevalent in the older population, and the major consequences of sleep-related breathing disorders such as sympathetic activation, nocturnal hypertension, and hypoxemia could impair LV diastolic function. It is, therefore, conceivable that sleep-related breathing disorders are a cause of diastolic dysfunction or contribute to its progression. Epidemiologic and therapeutic studies are needed to define the relationship of these two disorders, particularly in the older population, and the impact of treatment of sleep apnea on the natural history of isolated diastolic heart failure.
Sex and Sleep-Related Breathing Disorders in Systolic Heart Failure
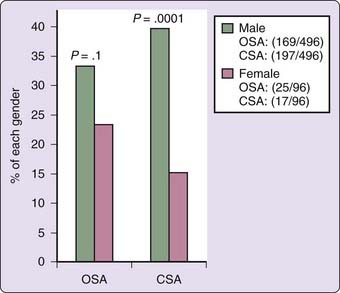
In the general population, the prevalence of OSA is significantly higher in men than in women. This also holds true for CSA in systolic heart failure. Combining the results of several studies of patients with systolic heart failure,12–14,17,18 40% of the male patients and 18% of the female patients have CSA (Fig. 122-3). A similar trend was found for OSA.
The results of population studies of subjects without heart failure (reviewed by Young and colleagues36) suggest that menopause may be a risk factor for OSA, and that the risk is probably reduced by hormone replacement therapy. In women with congestive heart failure and systolic dysfunction, the risk of CSA was six times higher in those aged 60 years or older than in those younger than 60 years.12 A similar difference was also reported for obstructive sleep apnea–hypopnea before and after age 60 years.12 Thus, female hormonal status plays a role in the development of sleep-disordered breathing in women with and without heart failure.
Progesterone is a known respiratory stimulant, and its effects on the respiratory system may in part explain the lower prevalence of central and obstructive sleep apnea in menstruating women. Progesterone increases ventilation37 and the tone of the dilator muscles of the upper airway.38 Furthermore, premenopausal women have a significantly lower apneic threshold than men.39 This should decrease the probability of developing central apnea during sleep in female subjects (see Mechanisms of Central Sleep Apnea, later).
Mechanisms of Sleep-Related Breathing Disorders in Heart Failure
Mechanisms of Central Sleep Apnea
The mechanisms of periodic breathing and central sleep apnea in heart failure are complex and multifactorial (see Chapter 100).5,40–42 In heart failure, alterations occur in various components of the negative feedback system controlling breathing that increase the likelihood of developing periodic breathing, during both sleep and wakefulness. In addition, there are specific sleep-related mechanisms that explain the genesis of CSA and the reason periodic breathing becomes so prevalent during sleep.
Mathematical models of the negative feedback system predict that increased arterial circulation time (which delays the transfer of information regarding changes in PO2 and PCO2 from pulmonary capillary blood to the chemoreceptors), enhanced gain of the chemoreceptors, and enhanced plant gain (e.g., decreased functional residual capacity) collectively increase the likelihood of periodic breathing.5,40–42
Delay in transfer of information plays a fundamental role in destabilization of a negative feedback system.11,43 It has the potential to convert a negative feedback system to a positive feedback system. In heart failure, arterial circulation time may be increased for a variety of reasons including dilation of cardiac chambers, increased pulmonary blood volume, and decreased cardiac output. However, patients with systolic heart failure invariably have increased circulation time. Therefore, although increased circulation time is necessary to develop periodic breathing, it does not explain why only some heart failure patients have periodic breathing. The second factor that increases the likelihood of occurrence of periodic breathing (and also central apnea during sleep) is the gain of the chemoreceptors.44 In persons with increased sensitivity to CO2 (or hypoxia), the chemoreceptors elicit a large ventilatory response whenever the PCO2 rises (or the PO2 decreases). The consequent intense hyperventilation, by driving the PCO2 below the apneic threshold, results in central apnea. As a result of central apnea, PCO2 rises (and PO2 falls) and the cycles of hyperventilation and hypoventilation (hypopnea) or central apnea are maintained.44 Differences in the gain of the chemoreceptors among patients with heart failure may in part explain why only some patients develop periodic breathing and central sleep apnea.
The third factor that may contribute to the development of periodic breathing in heart failure is decreased functional residual capacity, which results in underdamping.5,40–42 This means that for a given change in ventilation (e.g., a pause in breathing), changes in the controlled variables—namely PO2 and PCO2—will be augmented. In turn, the augmented changes in PO2 and PCO2 result in a pronounced compensatory ventilatory response, and overcompensation tends to destabilize breathing. Patients with heart failure may have decreased functional residual capacity for a variety of reasons, including pleural effusion, cardiomegaly, and decreased compliance of the respiratory system. Functional residual capacity may decrease further in the supine position, facilitating development of periodic breathing in this position.
The aforementioned mechanisms underlying periodic breathing are present during both sleep and wakefulness. However, in the supine position and during sleep, further changes, such as reduction in functional residual capacity, metabolic rate, and cardiac output, occur that will augment the likelihood of developing periodic breathing beyond that observed during wakefulness. Meanwhile, like obstructive apnea, central apnea usually occurs during sleep or when a subject is dozing. The genesis of CSA during sleep relates specifically to the removal of the nonchemical drive of wakefulness on breathing and to the unmasking of the apneic threshold—the level of PCO2 below which rhythmic breathing ceases.42,45 The difference between two PCO2 set points—the prevailing PCO2 minus the PCO2 at the apneic threshold, referred to as PCO2 reserve—is a critical factor for occurrence of CSA. The smaller this difference, the greater is the likelihood of occurrence of apnea (discussed later).
Normally, with the onset of sleep, ventilation decreases and PCO2 increases. As long as the prevailing PCO2 is above the apneic threshold, rhythmic breathing continues. However, in some patients with heart failure, the awake prevailing PCO2 does not significantly rise with onset of sleep.46,47 In addition, heart failure patients who develop central apnea have increased CO2 chemosensitivity below eupnea.46 Because of this and the proximity of the prevailing PCO2 to the apneic threshold, the likelihood of developing central apnea increases during sleep.
Several studies48–50 have shown that patients with heart failure and low arterial PCO2 have a high probability of developing central apnea during sleep. Predictive value of a low steady-state arterial PCO2 (<35 mm Hg) is about 80%.50 However, although an awake low arterial PCO2 is highly predictive of CSA, it is not a prerequisite. Many patients with heart failure and central sleep apnea have a normal awake arterial PCO2.50,51 What is important is the proximity of the apneic threshold to the arterial PCO2, and an increased CO2 chemosensitivity below eupnea.
Mechanisms of Obstructive Sleep Apnea
As noted earlier, obstructive sleep apnea and hypopnea are also common in heart failure. The mechanisms are multifactorial. First, periodic breathing resulting from systolic heart failure predisposes the susceptible subjects to develop upper airway occlusion during the nadir of the ventilatory cycles of periodic breathing.7,52 Second, increased venous congestion and pressure resulting from right heart failure may diminish upper airway size53 and facilitate upper airway occlusion. Venous congestion of the upper airway may be worse in the supine (than in the erect) position, and rostral fluid shift, particularly in the presence of edema in the lower extremities and redistribution of fluid into vascular space, may further compound upper airway patency. Third, patients with heart failure and OSA are commonly obese, and obesity may compromise upper airway patency. In summary, decreased upper airway size resulting from both venous congestion and obesity may predispose patients with heart failure to develop upper airway occlusion during the nadir of the ventilatory cycles of periodic breathing, when the tone of the dilator muscles of the upper airway decreases the most.
Pathologic Consequences and Prognostic Significance of Sleep-Related Breathing Disorders
The cycles of apnea–hypopnea and hyperpnea, both obstructive and central, are associated with three adverse consequences. These include intermittent arterial blood gas abnormalities characterized by hypoxemia–reoxygenation and hypercapnia–hypocapnia, excessive arousals and shift to light sleep stages, and large negative swings in intrathoracic pressure (for details, see Chapter 119). The pathophysiologic consequences of obstructive and central sleep apneas and hypopneas adversely affect various cardiovascular functions and are potentially most detrimental in the presence of established coronary artery disease and left ventricular systolic and diastolic dysfunction.
Effects of OSA on Cardiovascular Function and Mortality
In patients with systolic heart failure, the presence of OSA is associated with increased sympathetic activity and reduced left ventricular ejection fraction, which are reversed if sleep apnea is effectively treated with nasal CPAP.54–57 There are four randomized clinical trials55–58 of CPAP therapy for OSA in patients with systolic heart failure. In three of these studies,55–57 left ventricular ejection fraction increased significantly (when compared with the control group), by about 10%, 5%, and 2%. In two57,58 of these four studies, sham CPAP was used in the control group; in one,57 left ventricular ejection fraction increased significantly but slightly (by 2%) and in the other one,58 ejection fraction did not increase. In the latter study, auto-CPAP was used and the adherence hours to CPAP was less than in the two previous studies,54,55 which had demonstrated 10% and 5% increases in ejection fraction. The increase in left ventricular ejection fraction is important because it is a predictor of survival in patients with systolic heart failure.
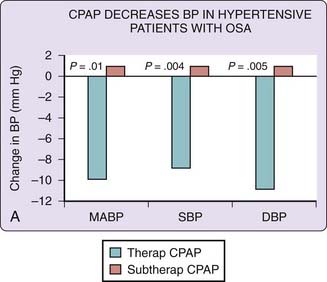
In patients with established coronary artery disease, obstructive sleep apnea is an independent prognostic factor for recurrent cardiovascular disorders and survival.59–61 Similarly, in systolic heart failure, two observational studies6,22 suggest that OSA contributes to mortality and that therapy with CPAP improves survival, particularly in those who are most compliant with it (Fig. 122-4).62 This latter observation in patients with heart failure is similar to that in patients with hypertension, as studies indicate that the reduction in blood pressure is most prominent in those who are most adherent to CPAP therapy (see Chapter 120).
Effects of CSA on Cardiovascular Function and Mortality
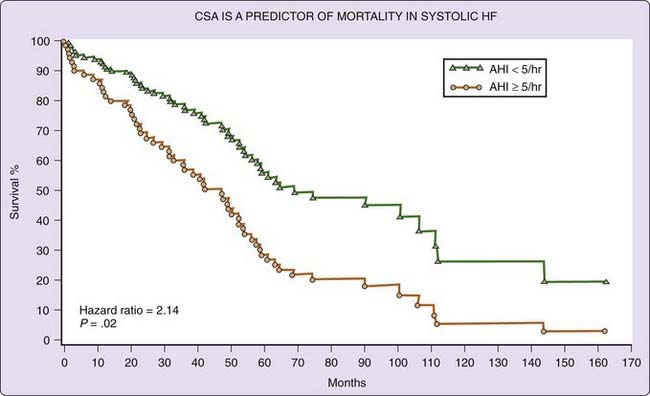
Like OSA, CSA is associated with increased sympathetic activity and reduced left ventricular ejection fraction, which was reversed by effective therapy with CPAP63 and oxygen (see later). Several studies,21,63–72 but not all,73,74 have suggested that presence of central sleep apnea decreases survival among patients with systolic heart failure. In one73 of the two studies73,74 noted, there was a tendency for excess mortality in heart failure patients with CSA, although this was not significant, probably because of the small number of patients.
We followed 88 heart failure patients with (n = 56) or without (n = 32) CSA with a median follow-up of 51 months.72 After controlling for 24 confounding variables, CSA was associated with excess mortality (hazard ratio, 2.14; P = .02; Fig. 122-5). The average survival of heart failure patients without CSA was 90 months compared with 45 months for those with CSA. That CSA contributes to excess mortality in systolic heart failure is supported by the observation that effective treatment of CSA with CPAP devices improves survival.71
Clinical Presentation of Obstructive and Central Sleep Apnea in Patients with Heart Failure
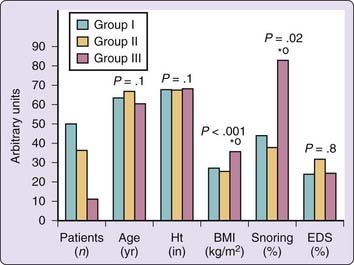
Obesity is an important risk factor for development of OSA in patients with heart failure,9,12,16 as it is for patients without heart failure.29 Patients with systolic heart failure and OSA are significantly heavier and snore habitually (Fig. 122-6). They may also have a higher systemic arterial blood pressure than subjects with CSA.9,12 Aside from obesity and habitual snoring, it is often difficult to clinically suspect the presence of sleep apnea in patients with heart failure because (1) the prevalence of sleepiness is similar in heart failure patients with and in those without sleep apnea9,16 (see Fig. 122-6), and (2) the symptoms of heart failure and sleep apnea overlap. The overlapping symptoms of sleep apnea and heart failure include sleep-onset and maintenance insomnia, nocturia, waking up with shortness of breath (orthopnea, paroxysmal nocturnal dyspnea, hyperpnea due to periodic breathing), unrefreshed sleep, and daytime fatigue. The overlapping of the symptoms of heart failure and sleep apnea undoubtedly contributes to the underdiagnosis of sleep-related breathing disorders in patients with heart failure. Central sleep apnea in particular is most difficult to diagnose,8 because obesity and habitual snoring, which are the two hallmarks of OSA (see Fig. 122-6), are commonly absent in heart failure patients with central sleep apnea.8,9 However, there are some clues that, when present, should increase the probability of the presence of CSA. These include a high-numbered class in the New York Heart Association classification, low left ventricular ejection fraction, and steady-state arterial PCO2, atrial fibrillation, and nocturnal ventricular arrhythmias (Fig. 122-7).9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree