INTRODUCTION
The hereditary ataxias comprise a wide spectrum of genetically determined disorders with ataxia as the prominent symptom. In most of these disorders, ataxia is due to degeneration of the cerebellar cortex and the spinal cord. The genetic classification of hereditary ataxias is given in Table 35.1.
AUTOSOMAL RECESSIVE ATAXIAS
The autosomal recessively inherited ataxias are a heterogeneous group of progressive ataxias. The majority of them are complex and disabling diseases in which ataxia is associated with involvement of other parts of the nervous system and of other organ systems. Typically, recessive ataxias start before the age of 20 years, but for most of these disorders, late-onset variants have been observed (1,2).
The precise number of recessive ataxias is difficult to define because ataxia is a frequent symptom of many recessive brain disorders, including disorders that are usually classified as storage disorders, leukodystrophies, or metabolic diseases. Thanks to advanced sequencing methods, several new autosomal recessive ataxias have been recently identified, and it is expected that their number will continue to increase. This chapter will focus on the most common forms and on those for which treatments are available. A comprehensive overview of all autosomal recessive ataxias can be found on http://neuromuscular.wustl.edu/ataxia/recatax.html.
FRIEDREICH’S ATAXIA
Friedreich’s ataxia (FRDA) is the most common autosomal recessively inherited ataxia in North America and Europe, but has not been described in Japan. Published prevalence rates show large variations in Europe ranging from less than 0.5 to almost 5 in 100,000 with a gradient from west to east (3–5).
| Genetic Classification of Hereditary Ataxias |
Autosomal recessive ataxias
Friedreich’s ataxia (FRDA)
Ataxia telangiectasia (AT)
Autosomal recessive ataxia with oculomotor apraxia type 1 (AOA1)
Autosomal recessive ataxia with oculomotor apraxia type 2 (AOA2)
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS)
Ataxia with isolated vitamin E deficiency (AVED)
Abetalipoproteinemia
Refsum’s disease
Cerebrotendinous xanthomatosis (CTX)
Other recessive ataxias
X-linked ataxias
Fragile X–associated tremor ataxia syndrome (FXTAS)
Autosomal dominant ataxias
Spinocerebellar ataxias (SCA)
Episodic ataxias (EA)
In most cases of FRDA, the causative mutation is a homozygous, intronic GAA repeat expansion in a gene coding for the mitochondrial protein frataxin. In healthy subjects, the repeat length ranges from 6 to 34, whereas FRDA patients have 67 to 1,700 repeats (6). There is an inverse relation between the repeat length and the disease phenotype with longer repeats associated with earlier onset and more severe symptoms (7). A small minority of FRDA patients are compound heterozygotes with one allele carrying the GAA repeat expansion and the other a point mutation (8).
The intronic GAA repeat expansion impedes normal transcription of the frataxin gene, resulting in markedly reduced frataxin tissue levels. Frataxin deficiency is more pronounced in patients with long repeats than in those with only moderate expansions. Frataxin is an iron-binding protein that assembles to larger protein complexes that are specialized in iron–sulphur cluster biogenesis. Frataxin deficiency results in a decline of mitochondrial respiratory activity, increased production of free radicals and mitochondrial iron overload (9).
The first pathologic changes in FRDA are thought to occur in the dorsal root ganglia with loss of large sensory neurons. In advanced cases, the neuropathologic abnormalities comprise axonal sensory and motor neuropathies, degeneration of spinal tracts (spinocerebellar tracts, posterior columns, pyramidal tract), and concentric hypertrophic cardiomyopathy affecting all chambers and the septum. There is only occasional involvement of the cerebellum with loss of Purkinje cells and moderate cerebellar atrophy (10).
The most prominent sign of FRDA is progressive ataxia, initially affecting gait and stance, and later also arm movements. Muscle reflexes of the legs are absent in about 90% of the patients. Approximately 80% of the patients have extensor plantar responses. With progression of the disease, distal wasting of the lower and upper extremities develops. Due to pyramidal involvement and muscle wasting, FRDA patients may have considerable weakness. Approximately half of the patients have skeletal deformities (scoliosis, pes cavus), which are due to muscle wasting starting in early life. Almost all patients have sensory disturbances with reduced vibration and position sense. All FRDA patients develop an ataxic speech disorder, usually within the first 5 years of the disease. Oculomotor disorders include square-wave jerks during fixation and reduced gain of vestibulo–ocular reflex, whereas gaze-evoked nystagmus or saccadic hypermetria are usually absent. Physical examination reveals pale disks in many FRDA patients. However, a loss of visual acuity is encountered in only 10% to 20% of the patients. Similarly, 10% to 20% develop sensorineural hearing problems. Nonneurologic features include cardiomyopathy and diabetes mellitus (7).
Magnetic resonance imaging (MRI) shows atrophy of the cervical spinal cord with only little shrinkage of the cerebellum. Nerve conduction studies reveal an axonal form of sensory neuropathy. Most patients have repolarization changes of the electrocardiogram. In approximately 60% of FRDA patients, echocardiography reveals a hypertrophic cardiomyopathy that is clinically often asymptomatic.
Mean age at onset is 15 years, ranging from 2 to more than 70 years depending on the repeat length. In approximately 15%, ataxia starts after the age of 25 years. The majority of these late-onset patients have retained muscle reflexes and often spasticity. FRDA is a progressive disease leading to disability and premature death. Patients with disease onset before the age of 14 years have a faster disease progression than patients with a later disease onset (11).
A genetic test demonstrating the GAA repeat expansion is widely available and is used to confirm a clinical diagnosis of FRDA. Genetic testing is particularly useful in atypical cases with preserved muscle reflexes and late disease onset.
There are currently no compounds that have a beneficial effect on ataxia (12). Physiotherapy and speech therapy are generally recommended. Patients with clinically relevant cardiomyopathy, diabetes mellitus, and skeletal deformities should receive standard medical treatment.
ATAXIA TELANGIECTASIA
Ataxia telangiectasia (AT) is an autosomal recessively inherited multisystem disorder caused by mutations of the ATM gene (13). Several hundred distinct mutations distributed over the entire gene have been reported. The ATM gene encodes a member of the phosphoinositol-3 kinase family. By phosphorylating a number of key substrates, ATM acts as an activator of the DNA damage response induced by DNA double-strand breaks (14). Despite intense research, the precise mechanisms underlying neurodegeneration in AT remain unresolved. However, there is accumulating evidence that an impaired response to oxidative stress and mitochondrial dysfunction play an important role.
There is atrophy of the cerebellum that mainly affects the cerebellar cortex of the vermis. The number of cerebellar Purkinje cells is reduced, and Purkinje cells show abnormal arborization and ectopic localization. In addition, there are degenerative changes of the spinal cord, including degeneration of the posterior and lateral columns and atrophy of the anterior horn. The peripheral nervous system may be involved with a demyelinating neuropathy.
The incidence of AT in the United States of America was estimated to be approximately 0.3:100,000 births (15). An Italian population-based study found a prevalence of 1.2:100,000 (3).
AT is clinically characterized by a combination of neurologic and nonneurologic symptoms. Cerebellar ataxia is the clinical hallmark of AT. Ataxia of gait and stance usually become apparent when the child has learned to walk. Other cerebellar symptoms, including dysarthria and ataxia of the upper extremities, develop in the further course of the disease. In addition, many patients have choreoathetosis and dystonia. Muscle reflexes are usually weak or absent. AT patients have a peculiar difficulty initiating saccades (oculomotor apraxia). Intellectual abilities are normal in the beginning of the disease. Later, there may be mild impairments that are partly secondary to the physical disability. In late disease stages, patients develop sensory disturbances with impaired vibration and positional sense and distal muscle wasting.
Approximately 60% of AT patients have immunodeficiency. The most frequent clinical manifestations are recurrent sinopulmonary infections. AT patients have a considerably increased risk of malignancies. Overall, one-third of AT patients develop a malignant disease during their lives. Before the age of 20 years, malignancies are mainly lymphoid. In older patients, solid tumors are more frequent. Telangiectasias develop after the onset of ataxia and are most frequently found in the lateral angles of the conjunctivae and the external earlobes.
AT usually begins at 2 to 4 years after the child has learned to walk. Most patients need wheelchairs at the age of 10 years. Twenty-year survival rate is approximately 50%. Life expectancy is low in patients with mutations causing total loss of protein (16).
A probable diagnosis of AT is made in patients with a typical clinical phenotype and elevated serum levels of α-fetoprotein. In vitro demonstration of increased radiosensitivity of lymphocytes is used as a diagnostic test. Alternatively, the diagnosis can be confirmed by demonstrating absence or deficiency of ATM protein of kinase function in blood cells (17). Genetic testing is possible, but is currently not routinely used due to the size of the gene and the diversity of mutations causing AT.
Effective therapies for the neurologic manifestation of AT are not available. Treatment of infections should be initiated early and maintained over a prolonged time. Treatment of malignancies is a particular problem because AT patients have increased sensitivity to radiation and chemotherapy. Therefore, conventional radiotherapy should be avoided, and chemotherapy should be administered only on an individual basis.
ATAXIA WITH OCULOMOTOR APRAXIA TYPE 1
Autosomal recessive ataxia with oculomotor apraxia type 1 (AOA1) is an autosomal recessively inherited ataxia that is caused by mutations of the aprataxin gene (18). In a cohort of 102 patients with autosomal recessive ataxia, AOA1 was the fourth most common form (19). Fibroblasts isolated from patients with AOA1 show increased sensitivity to reactive oxygen species and decreased repair of single-strand DNA breaks (20). AOA1 may be associated with muscular coenzyme Q10 deficiency (21).
AOA1 starts in children with a mean age of onset of 7 years. Besides ataxia and oculomotor apraxia, the clinical spectrum of AOA1 involves chorea, neuropathy, and mental retardation (22). With progression of the disease, neuropathy becomes more and more disabling. AOA1 patients survive in a severely disabled state into adulthood.
Characteristically, routine laboratory tests show hypoalbuminemia that increases with progression of the disease. A definite diagnosis of AOA1 is made by genetic testing.
ATAXIA WITH OCULOMOTOR APRAXIA TYPE 2
Autosomal recessive ataxia with oculomotor apraxia type 2 (AOA2) is an autosomal recessively inherited ataxia caused by mutations of the senataxin gene. Heterozygous mutations in the same gene underlie a rare autosomal dominant form of amyotrophic lateral sclerosis (ALS) (23). Senataxin is a DNA/RNA helicase involved in transcription termination. Senataxin loss is associated with defective repair of single-strand DNA breaks (24). In a cohort of 102 patients with autosomal recessive ataxia, AOA2 was the second most common form (19).
The mean age of onset of AOA2 is 14 years. Clinically, AOA2 is characterized by ataxia and polyneuropathy. Oculomotor apraxia is present in about half of AOA2 patients (25).
AOA2 patients share the laboratory finding of increased serum α-fetoprotein levels with AT, but lack increased radiosensitivity. A definite diagnosis of AOA2 is made by molecular genetic testing.
AUTOSOMAL RECESSIVE SPASTIC ATAXIA OF CHARLEVOIX-SAGUENAY
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is an autosomal recessive ataxia with a distinctive phenotype that has been initially observed in a restricted area in Quebec in Canada. ARSACS is due to deletions or point mutations in a large, single exon gene–encoding sacsin (26). Sacsin contains a heat-shock domain, which suggests that it subserves chaperone function (27).
The prevalence of ARSACS in the French-Canadian founder population in Quebec is 50:100,000. Following identification of the mutations, ARSACS patients were diagnosed in several European countries, Tunisia, Brazil, and Japan, suggesting a worldwide distribution.
ARSACS is characterized by the combination of progressive cerebellar ataxia and spasticity. Muscle reflexes are exaggerated, and plantar responses are extensor. With progression of the disease, the ankle jerks disappear and distal wasting of foot muscles develops. A highly characteristic ocular sign is the presence of prominent myelinated fibers radiating from the optic disk at fundoscopy.
ARSACS typically starts at the age of 1 to 2 years. On average, patients become wheelchair-bound around the age of 40 years.
Apart from cerebellar and spinal cord atrophies, MRIs show a characteristic linear hypointensity of the pons on T2- and FLAIR-weighted images (28). The diagnosis of ARSACS can be confirmed by demonstration of the deletion in the sacsin gene. There is no effective therapy for ARSACS.
ABETALIPOPROTEINEMIA
Abetalipoproteinemia is a rare, autosomal recessively inherited disorder characterized by onset of diarrhea soon after birth and slow development of a neurologic syndrome thereafter. The neurologic syndrome consists of ataxia, weakness of the limbs with loss of tendon reflexes, disturbed sensation, and retinal degeneration. Abetalipoproteinemia is caused by mutations in the gene encoding a subunit of a microsomal triglyceride transfer protein (29). As a consequence, circulating apoprotein B-containing lipoproteins are almost completely missing, and patients are unable to absorb and transport fat and fat-soluble vitamins. The neurologic symptoms are due to vitamin E deficiency. Management of abetalipoproteinemia consists of a diet with reduced fat intake and oral vitamin E supplementation (50–100 mg/kg per day).
ATAXIA WITH ISOLATED VITAMIN E DEFICIENCY
Ataxia with isolated vitamin E deficiency (AVED) is a rare, autosomal recessively inherited disorder with a phenotype resembling FRDA. AVED patients carry homozygous mutations of the gene encoding the α-tocopherol transport protein, a liver-specific protein that incorporates vitamin E into very low-density lipoproteins (30). As a consequence, vitamin E is rapidly eliminated. AVED is a frequent cause of recessive ataxia in North African countries but is rarely encountered in other parts of the world. Since there is no absorption deficit, oral supplementation of vitamin E at a dose of 800 to 2,000 mg/day is recommended.
REFSUM’S DISEASE
Refsum’s disease is a rare, autosomal recessively inherited disorder due to mutations in the gene encoding phytanoyl-CoA hydroxylase that is involved in the α-oxidation of phytanic acid (31). The clinical phenotype of Refsum’s disease is caused by accumulation of phytanic acid in body tissues. Clinically, Refsum’s disease is characterized by ataxia, demyelinating sensorimotor neuropathy, pigmentary retinal degeneration, deafness, cardiac arrhythmias, and ichthyosis-like skin changes. Whereas ocular and hearing problems are usually slowly progressive, there may be acute exacerbations that are precipitated by low caloric intake and mobilization of phytanic acid from adipose tissue.
Refsum’s disease is treated by dietary restriction of phytanic acid from 50 to 100 mg/day contained in a normal Western diet to less than 10 mg/day. With good dietary supervision, ataxia and neuropathy may improve. In contrast, the progressive loss of vision and hearing cannot be prevented. In acute exacerbations, plasma exchange is effective in lowering phytanic acid levels and improving neurologic and cardiac function.
CEREBROTENDINOUS XANTHOMATOSIS
Cerebrotendinous xanthomatosis (CTX) is a rare, autosomal recessively inherited lipid storage disorder. The disorder is due to mutations of the gene encoding sterol 27-hydroxylase (32). Sterol 27-hydroxylase is responsible for the degradation of 7a-hydroxy-cholesterol to bile acids. As a consequence of the enzymatic defect, there is reduced excretion of bile acids with feces, whereas the content of bile alcohols increases. In addition, cholesterol and 7a-hydroxy-cholesterol are increasingly degraded to cholestanol. It is assumed that CTX is caused by the accumulation of cholestanol.
CTX is a progressive multisystemic disorder. The neurologic syndrome includes ataxia, pyramidal signs, cognitive impairment, epilepsy, and peripheral neuropathy. In addition, patients have chronic diarrhea, xanthomatous tendon swelling, and cataracts. The tendon xanthomas that gave rise to the naming of the disorder are not obligate, so their absence does not rule out a diagnosis of CTX.
CTX is treated by oral administration of chenodeoxycholate (750 mg/day). Treatment can be further improved by addition of statins.
FRAGILE X–ASSOCIATED TREMOR ATAXIA SYNDROME
Fragile X syndrome (FXS) is the most common inherited form of mental retardation. It is an X-linked disorder caused by a CGG expansion (>200; normal: 6–44) in the 5′ region of the FMR1 gene, resulting in hypermethylation of the FMR1 promotor, transcriptional silencing, and loss of FMR1 protein. FMR1 premutations (200) are frequent in the general population and may give rise to full mutations. FXS-associated tremor ataxia syndrome (FXTAS) is a unique form of clinical involvement in male FMR1premutation carriers characterized by progressive action tremor and cerebellar ataxia (33). These symptoms may be accompanied by cognitive decline, parkinsonism, neuropathy, and autonomic failure. MRI show hyperintensities in the middle cerebellar peduncles and corpus callosum splenium (34). In contrast to FXS, FXTAS patients have increased levels of FMR1 mRNA and normal FMR1 protein levels.
Penetrance of neurologic impairment in male carriers of FMR1 premutations is incomplete (35). Screening for FMR1 premutations in male patients with unexplained ataxia yielded only a small number of positive results.
SPINOCEREBELLAR ATAXIAS
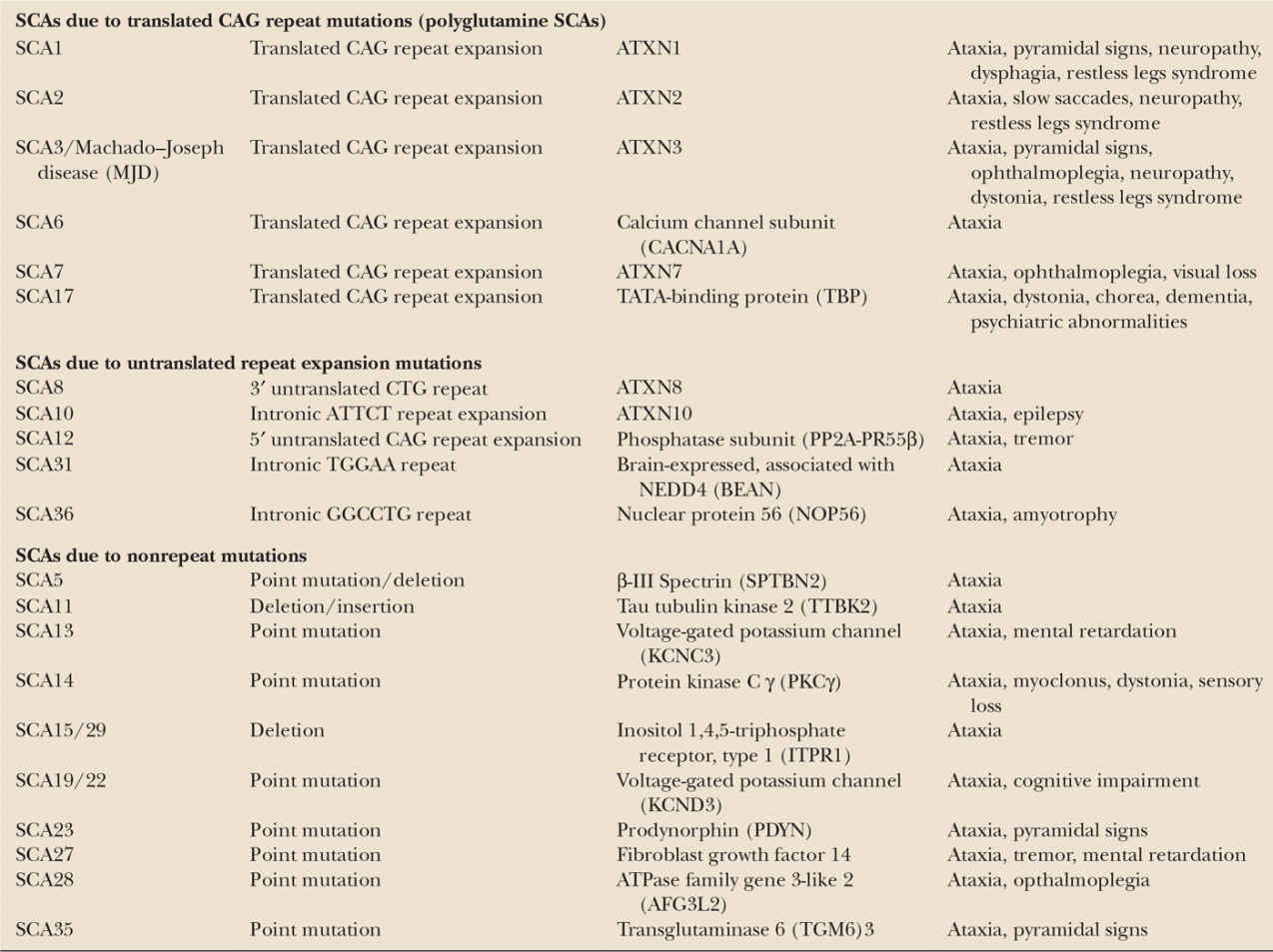
The spinocerebellar ataxias (SCAs) are a genetically heterogeneous group of more than 30 autosomal dominantly inherited progressive ataxia disorders (36). Genetically, they can be subdivided into three groups. SCA1, SCA2, SCA3, SCA6, SCA7, and SCA17 are caused by translated CAG repeat expansion mutations coding for an elongated polyglutamine tract within the respective proteins. These disorders belong to a larger group of polyglutamine disorders that also include Huntington’s disease. The second group comprises SCA8, SCA10, SCA12, SCA31, and SCA36, which are caused by untranslated repeat mutations. A third group comprises SCAs which are due to conventional nonrepeat mutations, mainly point mutations (37) (Table 35.2). A number of SCAs have been linked to a chromosomal locus, but the disease gene and causative mutations have not yet been found. Detailed information on all SCAs can be found on http://neuromuscular.wustl.edu/ataxia/recatax.html.
An epidemiologic study performed in the Netherlands found a prevalence of SCAs of 0.8–3.0: 100,000 (38). A study from Norway found a prevalence of 4.2: 100,000 (39). However, regional prevalence rates may be much higher due to founder effects.
The distribution among the various genetic subtypes varies from region to region. Worldwide, the polyglutamine SCAs are the most common SCAs and account for the disease in more than half of all SCA families (36).
SCA DUE TO TRANSLATED CAG REPEAT EXPANSION MUTATIONS (POLYGLUTAMINE SCAS)
With the exception of SCA6, all polyglutamine SCAs are multisystemic disorders with a clinical syndrome suggesting widespread involvement of the central and peripheral nervous system going beyond the cerebellum and spinal cord. Neurodegeneration is often most pronounced in the cerebellum and brain stem, resulting in a highly characteristic pattern of olivopontocerebellar atrophy. First clinical manifestations typically appear between the age of 30 and 40 years, again with the exception of SCA6 which starts about 20 years later. In all polyglutamine SCAs, there is an inverse correlation between the age of onset and repeat length (36). After disease onset, polyglutamine disorders take a steadily progressive course leading to considerable disability with years after disease onset and premature death.
< div class='tao-gold-member'>