Hydrocephalus
Michelle L. Ghobrial
Leon D. Prockop
Fred Rincon
INTRODUCTION
Hydrocephalus is characterized by an imbalance in the production, drainage, and reabsorption of cerebrospinal fluid (CSF) resulting in a dilation of the cerebral ventricles. The choroid plexus produces about 500 mL of CSF daily. The CSF circulates from the lateral ventricles to the third ventricle through the foramen of Monro. It then flows to the fourth ventricle via the cerebral aqueduct and exits into the subarachnoid space via the foramina of Magendie and Luschka. The arachnoid villi granulations reabsorb the CSF into the venous system.
CLASSIFICATION
Although various classifications of hydrocephalus have evolved historically, the several types are recognized (Table 106.1). Although all types of hydrocephalus are obstructive to some degree, the anatomic localization and severity of resistance to normal anterograde CSF flow can vary. Obstructive hydrocephalus is used to describe conditions that result near complete blockage of CSF flow within the ventricular system. Communicating hydrocephalus describes conditions in which the ventricles are enlarged despite an open flow system from within the ventricles into the basal cisterns and over the convexities. Walter Dandy first described communicating hydrocephalus in 1914. He injected a tracer dye into one of the lateral ventricles. If the dye appeared in lumbar CSF, the hydrocephalus was termed communicating; if the dye did not appear in lumbar CSF, the hydrocephalus was termed noncommunicating. Because it proved useful in surgical-shunt placement, this functional classification was widely accepted; however, by this definition, noncommunicating hydrocephalus refers only to that caused by obstruction within the ventricular system. Communicating hydrocephalus infers CSF obstruction due to damage to the absorptive system after exiting the ventricles. Normal pressure hydrocephalus (NPH) is a form of communicating hydrocephalus.
TABLE 106.1 Classification of Hydrocephalus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hydrocephalus can be acute or chronic. Acute hydrocephalus can be an immediate threat to life when it develops rapidly over several hours and leads to intracranial hypertension and downward central herniation of the brain. By contrast, even massive ventriculomegaly can be minimally symptomatic if it develops gradually over weeks, months, or years and does not represent a threat to life.
Another classification of hydrocephalus is congenital versus acquired. Congenital hydrocephalus is present at birth. Acquired hydrocephalus can develop any time after birth. These forms of hydrocephalus are distinguished from hydrocephalus ex vacuo in which CSF volume increases without change in CSF pressure because of cerebral atrophy.
EPIDEMIOLOGY
Population-based statistics for hydrocephalus are difficult to provide given the scope of varying ages of onset and causes. Congenital hydrocephalus occurs with an incidence of 0.5 to 1.8 per 1,000 births per year and may result from either genetic or nongenetic causes. Posthemorrhagic hydrocephalus occurs with an incidence of 25% to 70%, depending on the severity of the hemorrhage. Approximately 10% of infants with intraventricular hemorrhage (IVH) will require a shunt. The incidence of acute acquired hydrocephalus is unknown. Recent populationbased studies have estimated the prevalence of NPH to be about 0.5% in those older than 65 years, with an incidence of about 5.5 patients per 100,000 per year. This is in accordance with comparable findings stating that although NPH occurs in both men and women of any age, it is found more often in the elderly population, with a peak onset generally in the sixth to seventh decades. Low-pressure hydrocephalus is reported in the literature as case series but no population statistics are known due to its uncommon occurrence.
PATHOBIOLOGY
OBSTRUCTIVE HYDROCEPHALUS
In intraventricular obstructive hydrocephalus, the obstruction causes proximal dilation of the ventricles with preservation of
normal ventricular size distally. Obstruction may occur at the foramen of Monro, the third ventricle, the aqueduct of Sylvius, the fourth ventricle, or the fourth ventricular outflow foramina of Luschka and Magendie. Obstructive hydrocephalus can be caused by congenital malformations, developmental lesions, mass lesions, or posthemorrhagic ventricular obstructions.
normal ventricular size distally. Obstruction may occur at the foramen of Monro, the third ventricle, the aqueduct of Sylvius, the fourth ventricle, or the fourth ventricular outflow foramina of Luschka and Magendie. Obstructive hydrocephalus can be caused by congenital malformations, developmental lesions, mass lesions, or posthemorrhagic ventricular obstructions.
Congenital Malformations and Developmental Lesions
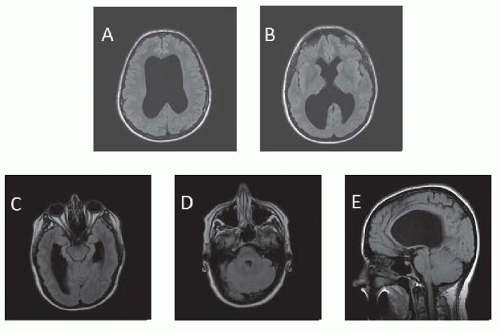
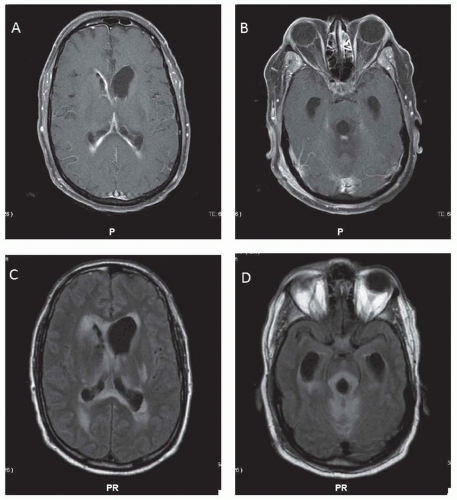
Common nongenetic causes include intracranial hemorrhage (ICH) secondary to birth trauma or prematurity and meningitis as a cause for congenital hydrocephalus. Genetically, at least 43 mutants/loci linked to hereditary hydrocephalus have been identified in animal models and humans. In some, aqueductal stenosis has been documented by magnetic resonance imaging (MRI) or at postmortem examination. It is not clear whether aqueductal lesions (e.g., gliosis or fibrosis) occur developmentally or are the residue of prior viral inflammatory disease contracted in utero or in early life (Fig. 106.1). In some families, the occurrence of aqueductal stenosis, hydrocephalus of undetermined anatomic type, and the Dandy-Walker syndrome in siblings of both sexes suggests other modes of inheritance. In the Dandy-Walker syndrome, there is expansion of the fourth ventricle and the posterior fossa with obstruction of the foramina of Luschka and Magendie (Fig. 106.2). The Arnold-Chiari malformation may be associated with hydrocephalus at birth or it may develop later.
Mass Lesions
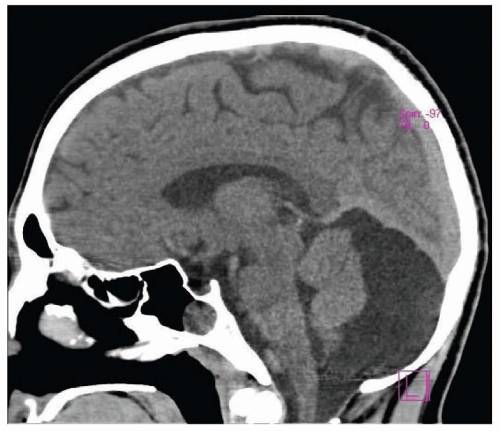
Intracranial neoplasms may cause obstructive hydrocephalus (Fig. 106.3). Tumors clustered about the third or fourth ventricles or the sylvian aqueduct, including pineal tumors, colloid cysts, gliomas, ependymoma, and metastases, are commonly implicated in intraventricular obstructive hydrocephalus. Prognosis, after shunting, is largely related to the type of tissue in the tumor. Other mass-like lesions, such as intraparenchymal cerebral hemorrhage, cerebellar infarction, or cerebellar hemorrhage, which assert local pressure on the ventricles may lead to acute hydrocephalus. Basilar artery ectasia and other vascular abnormalities (e.g., vein of Galen malformation) have also been associated with hydrocephalus.
Posthemorrhagic Ventricular Obstruction
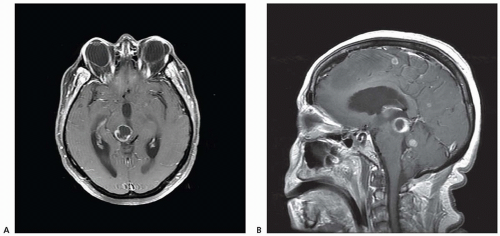
Intracerebral hemorrhage complicated by IVH in adults also causes hydrocephalus (Fig. 106.4). In some patients, CSF flow obstruction is transient; therefore, intracranial pressure (ICP) increases and hydrocephalus appears but then disappears spontaneously. Other patients exhibit progressive hydrocephalus.
COMMUNICATING HYDROCEPHALUS
When neither an intraventricular nor an extraventricular obstruction is documented, three other mechanisms may cause hydrocephalus: oversecretion of CSF, venous insufficiency, or impaired absorption of CSF by arachnoid villi. The absorptive capacity of the subarachnoid space is about three times the normal CSF formation rate of 0.35 mL/min (20 mL/h or 500 mL/day). Formation rates greater than 1.0 mL/min may produce hydrocephalus. Clinically, choroid plexus papilloma is the only known cause of oversecretion hydrocephalus.
Posthemorrhagic Hydrocephalus
Posthemorrhagic hydrocephalus is a major complication of cerebral IVH in low birth weight infants. Hydrocephalus results when a clot within the ventricular system obstructs CSF flow by a process of obliterative basilar arachnoiditis or by cortical arachnoiditis. Hydrocephalus also occurs in adults after subarachnoid bleeding caused by head trauma or ruptured aneurysm (see Fig. 106.4). After subarachnoid hemorrhage, distension of the arachnoid villi by packed red cells suggests an absorptive defect as a pathogenic mechanism for hydrocephalus. Consequently, fibrotic impairment of extraventricular CSF pathways after ICH may be complicated by dysfunction of arachnoid villi. In preterm infants, there is a predisposition to germinal matrix (GM) hemorrhage leading to periventricular hemorrhagic infarction (PHI) and IVH causing hydrocephalus. GM hemorrhage is graded by severity, as I through IV, whereby grades III and IV usually develop progressive hydrocephalus that requires shunting.
Postinfectious Hydrocephalus
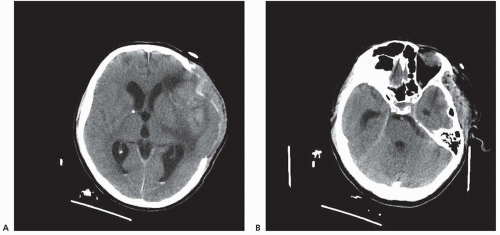
Among infectious diseases, bacterial, fungal, tubercular, or syphilitic meningitis may cause chronic hydrocephalus secondary to basal arachnoiditis (Fig. 106.5). Among parasitic infections, neurocysticercosis may produce both communicating and noncommunicating hydrocephalus.
Otitic Hydrocephalus
Otitic hydrocephalus occurs in children after chronic otitis media or mastoiditis with lateral sinus thrombosis. Otherwise, impaired cerebral venous drainage, for example, thrombosis of cortical veins or intracranial venous sinuses, rarely causes hydrocephalus. Rarely, hydrocephalus due to impairment of extracranial venous
drainage follows radical neck dissection or obstruction of the superior vena cava.
drainage follows radical neck dissection or obstruction of the superior vena cava.
Congenital Agenesis of the Arachnoid Villi
Communicating hydrocephalus has been attributed to congenital agenesis of the arachnoid villi with consequent impairment of CSF absorption. Because detailed pathologic study of the number of villi and their structural characteristics is difficult and rarely performed, this defect may be more common than statistics indicate. Likewise, dysfunction of arachnoid villi without obstruction of basilar or transcortical CSF pathways is not easy to assess.
Hyperproteinorraquia
Hydrocephalus has also been described when lumbar CSF protein content exceeds 500 mg/dL, as in polyneuritis or spinal cord tumor. The protein may interfere with CSF absorption. Ependymoma, the most common spinal cord tumor associated with hydrocephalus, may cause tumor seeding of the arachnoid villi.
Normal Pressure Hydrocephalus
NPH is a form of chronic communicating hydrocephalus with incomplete obstruction of the normal pathway of CSF flow. NPH most likely results from a serial pattern of increased resistance to normal CSF flow at the foramen of Monro, sylvian aqueduct, fourth ventricular outflow tracts, and the arachnoid granulations. The result is a relative increase in the volume of the lateral and third ventricles, with normal ICP. Symptoms are produced by stretching and secondary dysfunction and degeneration of white matter pathways in the corona radiata, which encase the lateral ventricles, and to a lesser extent white matter tracts in the internal capsule and below.
NPH is often “idiopathic” and may relate simply to an abnormal brain aging process, whereas in some cases, it may follow subarachnoid hemorrhage from trauma or aneurysm, meningitis, tumor, or surgery. Irrespective of the underlying etiology, the ventricles expand at the expense of brain volume, causing both brain compression and periventricular white matter changes. These changes are thought to arise from brain edema caused by transependymal flow of fluid (Fig. 106.6) or to ischemic demyelination caused by compression of brain tissue. There may also be neuronal dysfunction from the compression. However, the overall effect is sufficiently chronic, or compensated, so that the CSF pressure is normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree