Fig. 39.1
Hypothalamic hamartoma. (a) Sagittal T1-weighted pre-gadolinium MR image. (b) Coronal T1-weighted pre-gadolinium image. A well-circumscribed mass with slight T1 hyperintensity (arrow) is seen in the suprasellar region posterior to and abutting the pituitary stalk. The mass appears separate from the pituitary gland
39.3 Histopathology
These growths consist of abnormal distributions of histologically normal neuronal and glial cells [10].
They have an overall resemblance to hypothalamic gray matter [9].
Neuronal markers, such as synaptophysin, are typically positive.
Positive immunostaining for luteinizing hormone–releasing hormone (LHRH) has been demonstrated in tumors from children with precocious puberty [11].
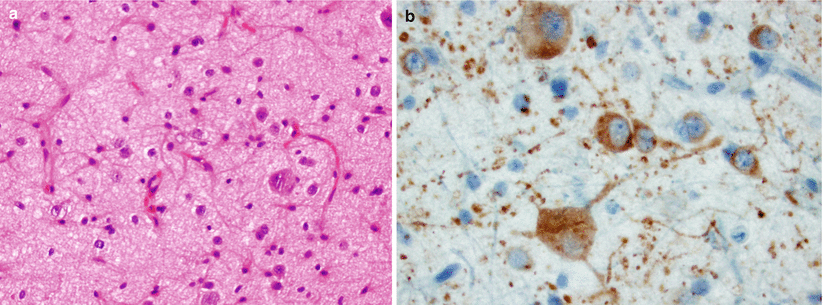
Fig. 39.2
Hypothalamic hamartoma. (a) Hypothalamic neuronal hamartoma is composed primarily of mature neurons arranged in a haphazard pattern or in small clusters. (b) The abnormally shaped neuronal cells are labeled with neuronal-associated markers, including microtubule-associated protein 2 (MAP2) (Courtesy of Dr. Carrie A. Mohila, Department of Pathology (Neuropathology), Texas Children’s Hospital, Baylor College of Medicine, Houston, TX)
39.4 Clinical and Surgical Management
Gonadotropin-releasing hormone (GnRH, LRH) analogues may be administered to successfully suppress central precocious puberty, making surgery for this particular indication somewhat obsolete [1, 12].
The primary indication for surgical treatment of hypothalamic hamartoma is seizures.
Video-EEG monitoring may demonstrate an associated epileptogenic cortical region that improves or normalizes following surgical resection [13, 14].
Interhemispheric–transcallosal–interforniceal and pterional approaches have been the most widely utilized open approaches for hypothalamic hamartomas [2, 14–16].
Complete seizure resolution has been reported in 49–54 % of patients following an interhemispheric approach, with another 24–35 % demonstrating some improvement in seizures [14–16].
Endoscopic approaches for resection or disconnection of hamartomas have been reported with excellent outcomes in recent years [8, 17, 18].
Complete resection via endoscopic intraventricular approaches has been reported in up to 32 % of patients, 93 % of whom were seizure-free at follow-up [17].
Hypopituitarism and diabetes insipidus occur frequently following surgical resection [1].
Short-term memory dysfunction may occur in up to 48 % of patients following surgical resection of hamartomas [14, 15].
In recent years, stereotactic radiosurgery has been utilized with a low risk profile and seizure improvement in 60 % of patients (complete seizure resolution in 40 % and reduced frequency in 20 %) [16].
Radiosurgery is more effective in treating smaller, noninvasive hamartomas, especially when the entire lesion can be covered [19–21].
Stereotactic radiofrequency thermocoagulation also has been reported to improve seizure frequency with some success for hamartomas that are small (<10 mm in diameter) [22].
References
1.
Freeman JL, Zacharin M, Rosenfeld JV, Harvey AS. The endocrinology of hypothalamic hamartoma surgery for intractable epilepsy. Epileptic Disord. 2003;5:239–47.PubMed
2.
Maixner W. Hypothalamic hamartomas–clinical, neuropathological and surgical aspects. Childs Nerv Syst. 2006;22:867–73.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








