and Robert E. Schmidt2
(1)
Sunnybrook and St Michael’s Hospitals, University of Toronto, Toronto, ON, Canada
(2)
Division of Neuropathology Department of Pathology, Washington University School of Medicine, St. Louis, MO, USA
Peripheral nerves are affected by a small number of infectious diseases in comparison to other organs. Nevertheless, the impact of these diseases is great, as evidenced by the frequency of leprous neuropathy in the underdeveloped world and HIV-related neuropathies globally. The spectrum of pathogens affecting the peripheral nervous system ranges from prions and viruses to spirochetes and parasites; some of these are reviewed in this chapter. Leprosy is discussed separately in Chap. 12. Parasitic diseases of the peripheral nervous system are discussed by Connor and Manz (1993).
11.1 HIV Infection
As understanding of the spectrum of disease associated with HIV infection has evolved over the last decade (Table 11.1), it has become clear that peripheral nerve pathology is present in nearly all patients by the end stage of their illness (i.e., AIDS), although it is often asymptomatic (Fuller et al. 1991, 1993; Gastaut et al. 1989; de la Monte et al. 1988). In the early stages of HIV infection, symptomatic neuropathy is seen in as few as 0.5 % of patients, but this increases dramatically as patients develop the full syndrome (Fuller et al. 1993; Barohn et al. 1993; So et al. 1988; Hall et al. 1991). Lange (1994), Gabbati et al. (2013), and Centner et al. (2013) provide a comprehensive review of the neuromuscular manifestations associated with HIV infection.
Table 11.1
Peripheral neuropathies associated with HIV infection
Distal symmetrical (predominantly sensory) polyneuropathy |
Inflammatory demyelinating polyneuropathy (GBS, CIDP) |
HIV-associated mononeuropathy multiplex (MM) syndromes |
Cytomegalovirus-associated neuropathies (lumbosacral polyradiculomyelopathy, CMV vasculitis) |
Herpes zoster reactivation |
Neuropathy associated with ddl/ddC |
Infiltrative (lymphomatous) neuropathy |
Diffuse infiltrative lymphocytosis |
Autonomic neuropathy |
The neuropathic syndromes seen in association with HIV infection tend to occur at specific phases in the evolution of the disease (Cornblath and McArthur 1988). During seroconversion or the subsequent asymptomatic stage, a neuropathy identical to Guillain–Barré syndrome (GBS) or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is sometimes seen. Mononeuropathy multiplex is an uncommon feature of the early symptomatic stage (ARC, CDC classes III and IVA) of HIV infection (Figs. 11.1 and 11.2). A distal symmetrical polyneuropathy (DSPN) is the most prevalent syndrome in patients with severe immunodeficiency (AIDS), who are also at risk of developing a neuropathy as the result of antiretroviral therapy with ddI (2′3′-dideoxyinosine) and ddC (2′3′-dideoxycytidine) (Table 18.1). Cytomegalovirus polyradiculoneuritis is increasingly recognized as a complication of HIV infection (Figs. 11.3 and 11.4), and lymphomatous neuropathies may also occur (Gold et al. 1988; Cohen et al. 1993; Fuller et al. 1993). Finally, autonomic neuropathy has been reported, typically characterized by orthostatic intolerance and secretomotor and gastrointestinal complaints, late in the course of HIV (Compostella et al. 2008).
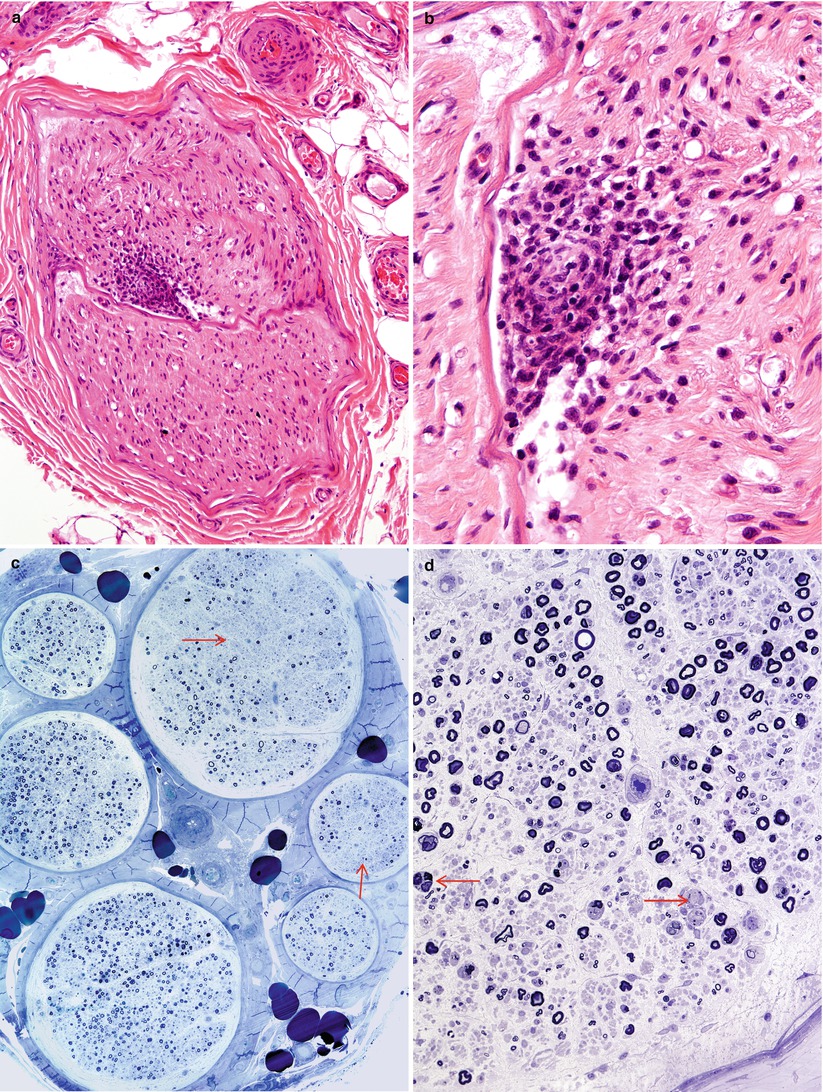
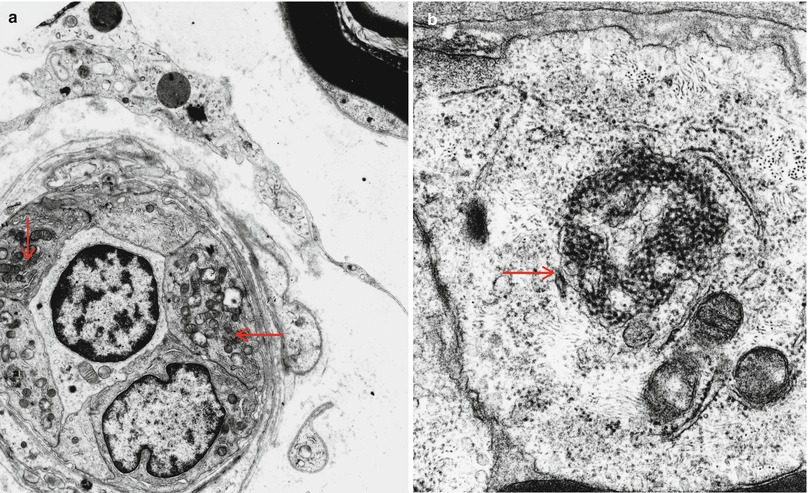
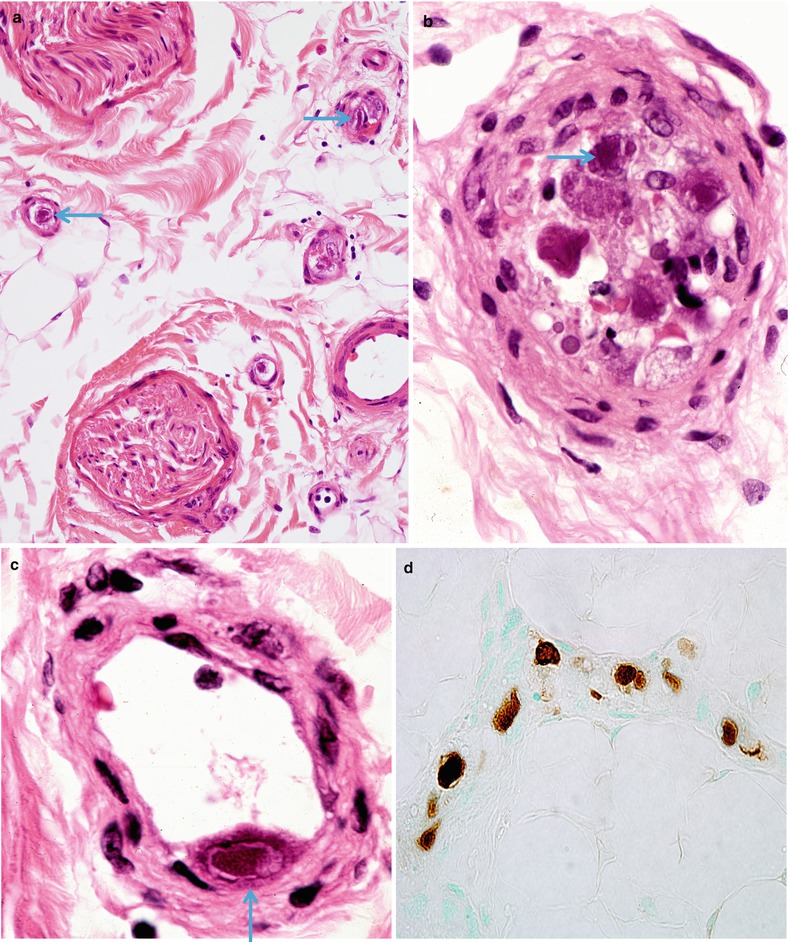
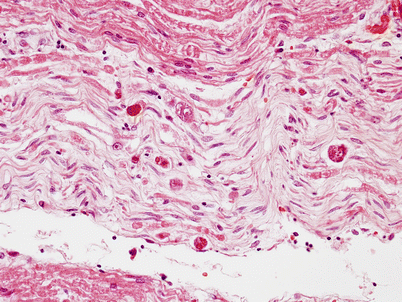

Fig. 11.1
HIV-associated vasculitis: An endoneurial microvessel is obliterated and surrounded by mononuclear cells. Note karyorrhexis in inflammatory infiltrate (b). Massive axonal degeneration with fascicle-to-fascicle variability in axon loss with intrafascicular loss (arrows, c) and numerous actively degenerating axons (arrows, d) is consistent with ischemic injury (c). Rows of myelin ovoids are seen in (d) (a, b, 400×, 1,000×; c, 200×; d, 400×) (a, b, paraffin H&E; c, 1 μ thick plastic section 400×; d, 1 μ thick plastic section 400×)

Fig. 11.2
HIV-associated vasculitis: Tubuloreticular inclusions (TRIs) (arrows a, b) are commonly found in endothelial cells of patients with AIDS, even in uninvolved vessels (a, 5,720×; b, 30,420×)

Fig. 11.3
CMV neuritis: Epineurial vessels (a: arrow) display typical amphophilic inclusions in endothelium (arrows, a–c). Lack of inflammation is attributed to immune suppression. (d) CMV is identified by immunostaining (a, 400×; b, c, 1,000×; d, 600×)

Fig. 11.4
Lumbosacral polyradiculomyelopathy: Extensive involvement of proximal portion of nerve root adjacent to lumbosacral cord represents an additional site of involvement (paraffin section, 600×)
Inflammation with CD8+ prominence has been described as “diffuse infiltrative lymphocytosis” syndrome (DILS) and may be confused with lymphoma in some cases. The former condition is characterized by hyperlymphocytosis with multivisceral CD8+ T-cell infiltration, particularly involving the salivary glands resulting in xerostomia. Additionally, there is marked angiocentric inflammation of nerve without angionecrosis (Moulignier et al. 1997). Mycobacterium avium intracellulare (MAI) has been reported within macrophages in peripheral nerve on two occasions (Cornblath et al. 1993), but whether it played a pathogenic role is unclear. Patients with underlying infectious neuropathy may develop IRIS (immune reconstitution inflammatory syndrome, Beishuizen and Geerlings 2009) which may present as acute inflammatory demyelinating polyneuropathy, i.e., as Guillain–Barré syndrome.
11.1.1 Distal Symmetrical Polyneuropathy (DSPN)
11.1.1.1 Clinical Manifestations
DSPN is seen in 10–35 % of patients in the late stages of HIV disease (Fuller et al. 1993; Barohn et al. 1993; Hall et al. 1991; Cornblath and McArthur 1988; So et al. 1988) but may occur in any stage of HIV infection (Vital et al. 1992; Hall et al. 1991; Winer et al. 1992). Initial complaints usually involve the legs distally, with paresthesias, sensory deficit, and dysesthetic pain sometimes so severe that patients are unable to walk because of discomfort rather than weakness. Motor involvement, if present, is usually confined to the distal lower extremity. Patients often have concomitant brain and spinal cord disease. The CSF may show a mild nonspecific elevation in cell count or protein, and electrical testing demonstrates diffuse distal axonal dysfunction with variable conduction slowing. Therapy is limited to symptom control (Cornblath et al. 1993).
11.1.1.2 Pathology
Although DSPN is by far the most common cause of neuropathy in this group of patients, other neuropathies must be considered, especially in patients experiencing weight loss and nutritional deficits. Patients with a clinical syndrome resembling DSPN may have necrotizing peripheral nerve vasculitis and are discussed separately below.
Light Microscopy
Epineurial or endoneurial inflammatory infiltrates may be seen. When present they are usually perivascular, range from minimal to very prominent, and do not correlate with the clinical severity, or even presence, of the neuropathy (Bailey et al. 1988; Chaunu et al. 1989; de la Monte et al. 1988; Fuller et al. 1993; Kiprov et al. 1988; Gastaut et al. 1989; Vital et al. 1992; Winer et al. 1992). Absence of inflammation may be related to systemic depletion of lymphocytes, as DSPN during AIDS-related complex (ARC) is more likely to show lymphocytic infiltration than DSPN during AIDS (Chaunu et al. 1989; Leger et al. 1988). Perivascular cuffing has been referred to as “microvasculitis” (Chaunu et al. 1989; Leger et al. 1988; Leport et al. 1987), but in our opinion, this is overstated in the absence of vessel necrosis or endothelial damage. Winer and colleagues (1992) observed frequent positivity for iron (Perls’ stain) in endoneurial and perineurial macrophages of patients with neuropathy. Such a finding might suggest a vasculitic process (Adams et al. 1989), but similar positivity was seen in relatively normal nerves, making this finding hard to interpret. Overall, although perivascular inflammation can be very prominent in DSPN, true vasculitis does not appear to be a significant factor.
Typically, the neuropathic process is a variably severe acute and chronic axonal degeneration (Fig. 11.5), with less prominent segmental demyelination (Gastaut et al. 1989; Kiprov et al. 1988; Winer et al. 1992; Bailey et al. 1988; Vital et al. 1992). Large myelinated fibers may be more involved (Gastaut et al. 1989), although this could reflect axonal atrophy rather than true selective loss of large axons (Fuller et al. 1990a). Unmyelinated fibers may be relatively spared (Fuller et al. 1990a). Regenerating clusters are occasionally seen. Segmental demyelination has not been a significant feature in studies employing morphometric techniques or electron microscopy (Mah et al. 1988; Cornblath et al. 1993; Vital et al. 1992), and the one report suggesting that segmental demyelination occurred in DPSN was performed without semithin sections, electron microscopy, or fiber teasing (de la Monte et al. 1988).
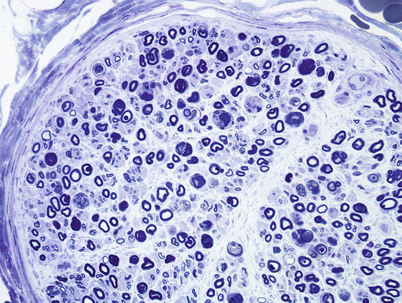

Fig. 11.5
HIV-associated Distal sensory polyneuropathy (DSPN): Extensive loss of myelinated axons with ongoing axonal degeneration may present with sensory loss and pain, often with increased numbers of macrophages (1 μ thick plastic section 400×)
Vital et al. (1992) found positive in situ hybridization for HIV in 2 of 12 patients with DSPN, but the cell containing the signal was not identified and could simply have been a circulating leukocyte. An immunohistochemical search for HIV antigens in peripheral nerve of patients with DSPN has been unrewarding (de la Monte et al. 1988; Grafe and Wiley 1989).
Electron Microscopy
Tubuloreticular inclusions (TRIs, vide infra) are probably detectable in most patients with DSPN (Mezin et al. 1991; Vital et al. 1992; Fuller and Jacobs 1989). TRI numbers seem to correlate with the duration and severity of HIV disease, as they were found in only 1 of 10 patients with ARC or HIV seropositivity and in 17 of 18 patients with AIDS (Mezin et al. 1991; Vital et al. 1992 ). TRIs are also detectable in macrophages and perineurial cells (Mezin et al. 1991). Mezin et al. (1991) observed necrosis of capillary endothelial cells in 5 of their 12 specimens, but no nearby inflammatory cells were identified (Mezin et al. 1991). Nonspecific basal lamina thickening may be seen.
Intra-axonal “retroviral-like” particles which were felt to be mature HIV nucleocapsids have been reported in nerve in only one case (Bailey et al. 1988). In autopsy studies, Schwann cells containing CMV inclusions were seen, but clinical details of the peripheral neuropathy were not provided (Grafe and Wiley 1989; Guarda et al. 1984). Inclusions consistent with CMV have not been described in typical cases of DSPN. Fuller and co-workers found axonal atrophy to be a prominent feature of painful DSPN (Fuller et al. 1990a).
11.1.1.3 Pathogenesis
Examination of the peripheral nervous system at autopsy in 27 patients dying of AIDS, with and without neuropathy, demonstrated axonal loss and Wallerian degeneration in all, with a proximodistal gradient (Cornblath et al. 1993). Distal degeneration of the fasciculus gracilis in patients with DSPN and AIDS has also been demonstrated (Rance et al. 1988). Such observations provide evidence for a distal axonopathy. Spinal ganglion disease cannot be implicated as the sole explanation for DSPN (Fuller et al. 1993) because there is a motor component to the neuropathy and because inflammation and cell loss at this site do not correlate with peripheral nerve axon loss (Henin et al. 1990; Cornblath et al. 1993). The significance of perivascular cuffing seen in many patients with DSPN is unclear because this is a nonspecific finding which can be prominent in asymptomatic patients (de la Monte et al. 1988).
Fuller and colleagues report a higher incidence of active or recurrent CMV infection in AIDS patients with DSPN (80 %) than AIDS patients without DSPN (37 %), suggesting a possible contributory role of CMV to DPSN (Fuller et al. 1993). However, other workers have not detected this correlation (Winer et al. 1992). That this form of neuropathy is not seen in immunosuppressed transplant patients suggests that the HIV virus itself plays a role. AIDS patients with and without DSPN have a similar nutritional state (Fuller et al. 1993).
The evidence for necrotizing vasculitis is weak in DPSN, but the observation of capillary endothelial cell necrosis in association with TRIs (Mezin et al. 1991) is intriguing. We have examined biopsies in HIV-associated and SLE-associated vasculitic neuropathy where numerous TRIs and necrotic endothelial cells were present (Case 11.1). Interferon administration, known to induce formation of TRIs, may cause a peripheral neuropathy, but its pathological substrate is not known (Gastineau et al. 1989).
The HIV has on rare occasions been isolated from nerve homogenates (Ho et al. 1985; de la Monte et al. 1988) or detected by in situ hybridization (Vital et al. 1992), most likely originating from infected leukocytes or macrophages circulating through the peripheral nerve (Gherardi et al. 1989). Indeed, viral antigen was not detected by immunohistochemistry in the same nerve specimens from which it was cultured (de la Monte et al. 1988). Nevertheless, it is possible that DSPN results from HIV infection (Cornblath et al. 1993), perhaps through neurotoxic substances secreted by HIV-infected phagocytes (Giulian et al. 1990) or interference with nerve function by binding of HIV gp120 glycoprotein to peripheral nerve or sensory neuron antigens (van den Berg et al. 1992; Apostolski et al. 1993).
11.1.2 Inflammatory Demyelinating Polyneuropathy in HIV Infection
11.1.2.1 Clinical Manifestations
Early in the HIV epidemic, GBS and CIDP received considerable attention (Cornblath et al. 1987). However, more recent prospective studies suggest that the incidence of these neuropathies in HIV infection is small (Barohn et al. 1993; Hall et al. 1991; Fuller et al. 1993). While these neuropathies tend to occur in seroconverting or asymptomatic HIV seropositive patients, they have been described in patients with ARC (Cornblath et al. 1987; Vital et al. 1992) and AIDS (Chaunu et al. 1989). The disease manifestations, including course and response to treatment, are identical to those seen in HIV seronegative patients, excepting only the presence of a CSF pleocytosis in many cases (Cornblath et al. 1987; Miller et al. 1988).
11.1.2.2 Pathology
The cardinal histological findings, mononuclear inflammation, macrophage-mediated myelin stripping, and a variable amount of axonal damage, are identical to those seen in inflammatory demyelination not associated with HIV (see Chap. 9) (Vital et al. 1992). The only observation that suggests an inflammatory demyelinating neuropathy is associated with HIV infection is visualization of tubuloreticular inclusions (TRIs) on electron microscopy (Fuller and Jacobs 1989). TRIs have not been reported in GBS or CIDP in the absence of HIV infection. Gibbels and Diederich (1988) described unusual “onion-bulb” formations in which the surrounding Schwann cell processes were thick and electron dense, and occasionally multinucleated! However, similar observations have not been reported elsewhere. As with DSPN, the virus has rarely been isolated from, but not directly visualized in, nerve in patients with this syndrome.
11.1.2.3 Pathogenesis
HIV has not been localized to endoneurial cells in these patients, and as the neuropathy tends to improve, it seems unlikely that the virus is directly related. Complement fixing anti-peripheral nerve antibodies similar to those described in classic GBS have been described in HIV seropositive patients with a GBS-like syndrome (Mishra et al. 1985). Kiprov et al. (1988) also reported patients with circulating anti-peripheral nerve antibodies, but identical findings were seen in HIV-infected patients with mononeuritis multiplex and DSPN. Patients are usually not severely immunosuppressed at the time of onset of their inflammatory demyelinating neuropathy, and most likely the pathogenesis of GBS and CIDP in HIV seropositive patients is similar to that of HIV seronegative patients (Chap. 9). The virus may play a role comparable to CMV or EBV in “precipitating” the onset of GBS.
11.1.3 Mononeuropathy Multiplex and Vasculitic Neuropathy in HIV Infection
11.1.3.1 Clinical Manifestations
Mononeuropathy multiplex (MM) has been described in HIV patients, most often during the early symptomatic (ARC) phase. The pathological substrate includes vasculitis associated with CMV infection (discussed separately below), vasculitis associated with HIV infection, and patients in whom no evidence of vasculitis is detected. Whether these groups truly differ or simply reflect the error inherent in sampling a nerve biopsy is unknown.
The patients with MM reported by Lipkin et al. (1985) did not have evidence of necrotizing vasculitis in nerve biopsy specimens, and often improved spontaneously. So and Olney (1991) also indicated that patients with mononeuritis multiplex tend to improve spontaneously or stabilize, but the histology in these patients was not specified.
Histologically verified non-CMV-related necrotizing vasculitis is a well-recognized complication of HIV infection (Calabrese et al. 1989) and can present neurologically as mononeuritis multiplex or distal symmetrical polyneuropathy (Case 11.1 below; Said et al. 1987; Dalakas and Pezeshkpour 1988; Gherardi et al. 1989; Kiprov et al. 1988; Conri et al. 1991; Lange 1994). Steroids or plasma exchange may be of benefit (Conri et al. 1991).
11.1.3.2 Pathology
Vasculitis involving endoneurial or epineurial vessels has been documented on many occasions in patients with HIV infection (Calabrese et al. 1989; Gherardi et al. 1989; Dalakas and Pezeshkpour 1988; Said et al. 1988a; Vinters et al. 1988; Weber et al. 1987; Lange 1994) and is probably underdiagnosed because these patients tend not to undergo biopsy. Issues relating to necrotizing vasculitis are discussed in detail in Chap. 13. In the one case of HIV-associated vasculitis we have examined, the vascular involvement took the form of endoneurial vascular and perivascular inflammation with karyorrhexis (Fig. 11.1a, b) with accompanying fascicle to fascicle variability in axon number and presence of large numbers of degenerating axons (Fig. 11.1d). Tubuloreticular inclusions were very prominent in this specimen (Fig. 11.2a, b), a finding which should always raise the alert for HIV infection, but we have also detected these in a patient with SLE and predominantly endoneurial vasculopathy (see Case 13.1).
The patients with mononeuropathy multiplex reported by Lipkin and colleagues (1985) showed perivascular inflammatory infiltrates, axonal degeneration, and demyelination of variable severity. No definite vasculitis was seen, but this does not rule out necrotizing vasculitis, which may be seen only on examination of another tissue or on angiography as demonstrated in other HIV-positive patients (Lange et al. 1988; Conri et al. 1991).
The HIV virus itself has been found in perivascular cells in vasculitis, probably macrophages, by techniques ranging from electron microscopy to RNA probes (Gherardi et al. 1989).
11.1.3.3 Pathogenesis
HIV antigens have been found in vessel walls and perivascular inflammatory cells in patients with vasculitis (Gherardi et al. 1989; Said et al. 1987). Circulating immune complexes to the HIV are present in seropositive patients (McDougal et al. 1985), a relevant observation in light of the known relationship between immune complexes and polyarteritis nodosa (Cornblath et al. 1993). Patients infected with HIV have also rarely been shown to suffer from angiocentric lymphoproliferative diseases which range from prominent polyclonal perivascular inflammation, through lymphomatoid granulomatosis, to malignant angiocentric lymphoma (Calabrese et al. 1989).
Case 11.1
A 34-year-old man was seen because of worsening paresthesia. He had tested positive for HIV infection 5 years earlier and had experienced intermittent diarrhea and a 40 lb weight loss a year prior to the neurological assessment. During the 6 months before nerve biopsy, the patient complained of paresthesias and numbness, involving first the left hand in a median nerve distribution, followed by the lateral left foot and shin, and then the right hand. Weakness of right hand muscles then ensued. Examination disclosed motor weakness in the right hand consistent with anterior interosseous nerve palsy. Discrete areas of diminished pin and temperature sensation were present in both the hands and feet, with bilateral mild impairment of distal vibration sensation at the toes. Reflexes were easily obtained and symmetrical. The patient had previously been treated with AZT, DDI, and DDC, but these had been discontinued prior to the onset of his symptoms due to adverse effects other than neuropathy.
At the time of neurological assessment, the absolute CD4 count was 34. ESR, ANA, immunoelectrophoresis, and routine biochemical and hematologic indices were normal. Electrophysiological tests performed on two occasions were interpreted as essentially normal, although a suspicion of bilateral carpal tunnel syndrome was entertained because of a slight disparity between distal latencies of the ulnar and median motor conductions. Electromyography was normal.
Nerve biopsy was performed 6 months after the onset of symptoms (Fig. 11.1). At this time, the patient was receiving low-dose steroid treatment for palliation of other symptomatology. Following nerve biopsy, no further treatment was offered, and the neuropathy continued to progress (clinical material courtesy of Dr. J. R. Wherrett).
11.1.4 Cytomegalovirus-Associated Neuropathy
The presence of CMV in peripheral nerve has been frequently documented, almost invariably in the setting of late-stage HIV infection (Bishopric et al. 1985; Eidelberg et al. 1986; Said et al. 1991; Fuller et al. 1990b; Morgello and Simpson 1994; Lange 1994). The clinical picture is usually fulminant polyneuropathy, polyradiculopathy, or mononeuritis multiplex. Some of these patients have improved with specific anti-CMV therapy (Said et al. 1991; Fuller et al. 1990b). CMV neuritis is probably part of the spectrum of CMV myeloradiculoneuritis (Cohen et al. 1993).
11.1.4.1 Pathology
The significance of finding CMV in any tissue in an AIDS patient is uncertain because this organism is ubiquitous by the end stages of the HIV infection (de la Monte et al. 1988; Guarda et al. 1984).
We have seen several cases of CMV-related vasculitic neuritis in an AIDS patient and another with immunosuppression in a cardiac transplant patient. The literature documents several patients with biopsy-proven CMV-associated peripheral nerve vasculitis, all with late-stage HIV infection (Said et al. 1991; Fuller et al. 1990b; Eidelberg et al. 1986). Nerve biopsy reveals necrotizing endoneurial and epineurial vasculitis with a prominent neutrophilic infiltrate, but in some cases, the tissue reaction is minimal, depending presumably on the host’s immune state (Fig. 11.3a). Cytomegalic cells, 30–50 μm in diameter, and cells containing intranuclear and intracytoplasmic amphophilic inclusions characteristic of CMV infection are observed, with immunostains confirming the presence of CMV (Fig. 11.3b–d). CMV virions can be identified on electron microscopy in endoneurial cells, especially endothelial cells. Although segmental demyelination can be present, it is overshadowed by active axonal degeneration.
CMV infection may also produce peripheral nerve disease through infection of Schwann cells. In one report, Schwann cell infection with CMV was demonstrated in four of seven patients with focal peripheral nerve inflammation; the infected cells were seen in association with regions of inflammation (Grafe and Wiley 1989). Clinical details were unfortunately not provided. Other reports document patients with a fulminant neuropathy and CMV inclusions in Schwann cells and other endothelial cells, associated with inflammation, demyelination, and cellular “necrosis” (Bishopric et al. 1985; Budzilovich et al. 1989). Rare cytomegalic Schwann cells containing intranuclear inclusions were associated with nerve root segmental demyelination in the case reported by Moskowitz et al. (1984). Morgello and Simpson (1994) reported a patient with multifocal demyelinating neuropathy where autopsy material demonstrated no evidence of vasculitis, but rather a mixed axonal and demyelinating neuropathy with evidence of Schwann cell necrosis. Intraneural mixed inflammatory infiltrates were associated with cytomegalic cells and “owl’s eye” inclusions, and herpesvirus capsids were demonstrated within Schwann cells.
Patients presenting with lumbosacral polyradiculomyelopathy, typically a late complication of CMV infection in AIDS, may have extensive involvement in the roots (Fig. 11.4) and adjacent spinal cord.
11.1.4.2 Pathogenesis
CMV neuritis seems to occur only in the setting of severe immunosuppression and can affect the peripheral nerve either by necrotizing vasculitis or through injury to Schwann cells and other endoneurial components.
11.1.5 Role of Nerve Biopsy in HIV Patients with Neuropathy
Little justification exists for nerve biopsy in typical cases of HIV-associated DSPN. The inflammatory demyelinating polyneuropathies (IDP) and vasculitic neuropathy seen in HIV disease may be responsive to specific treatment (vide supra) and should be identified. However, while biopsy might support the diagnosis of HIV-associated CIDP or GBS, histological examination is probably not very sensitive (Chap. 9), and in HIV infection, neural inflammation is nonspecific. Thus, clinical and electrophysiological criteria are probably more reliable than biopsy in making a diagnosis of CIDP or GBS in HIV patients.
The possibility of vasculitic neuropathy, often but not invariably associated with CMV infection, provides the strongest impetus for nerve biopsy in the HIV-positive patient, as this represents one of the few treatable neuropathies of HIV disease. CMV can also cause a demyelinating neuropathy (vide supra). The clinical picture may not always suggest the underlying process, because a predominantly sensory and symmetrical neuropathy, similar to DSPN, can be seen in this setting (Gherardi et al. 1989; Said et al. 1987; Calabrese et al. 1989). If mononeuritis multiplex is present, vasculitic neuropathy is suspected clinically, but the differential diagnosis includes HIV vasculitis, CMV vasculitis, or multiple peripheral nerve or root lesions due to lymphoma (Gold et al. 1988), CMV, or herpes zoster.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








