Clinical Aspects
Do not miss the diagnosis of an infectious disease. No matter what you call a glioblastoma, the patient will more than likely die within a year. But call an abscess a glioblastoma and the patient could be dead in weeks rather than in many years. For this reason, always ask yourself: “Could this lesion be infectious?” Ring-enhancing lesions could be abscesses or toxoplasmosis rather than glioblastomas or lymphomas.
In many cases, clinical data give clues that an infection underlies a brain lesion. The diagnosis could be obvious: the patient has a fever and a new focal neurologic deficit. Lesions in immunocompromised patients are always infectious until proven otherwise. In AIDS patients, this is well established. However, immunocompromised patients also include people who have undergone a transplant, have an underlying malignancy (e.g., chronic lymphocytic leukemia), or are on steroids for chronic obstructive pulmonary disease.
Obtaining a biopsy of a suspected infectious process has several purposes. Foremost is to confirm that the lesion is indeed infectious and not a tumor or an infarct. Knowing that the pathology is infectious, the main objective becomes identifying the etiologic agent. In most cases, an intraoperative culture will best identify the bug. Some infectious agents cannot be easily cultured (e.g., toxoplasmosis, JC virus), which then requires immunologic, molecular, or pathologic examination. Because most physicians are reluctant to insert needles in their patient’s heads, only those cases that require tissue will undergo biopsy. The diagnosis of most infectious diseases is based on clinical information or examination of the cerebrospinal fluid. Hence, most cases of meningitis, fungal infection, Herpes encephalitis, neurosyphilis, and tuberculosis meningoencephalitis never reach the pathologist’s microscope. As diagnostic polymerase chain reaction (PCR) techniques become more refined, infectious disease biopsies will continue to dwindle in number.
Like intraoperative examinations in all brain biopsies, in a possible infectious disease the primary purpose is to verify the presence of diagnostic tissue. A systematic assessment of the tissue is the key to the correct answer. First, what do the scans show? Many infectious diseases produce ring-enhancing lesions. Some give a meningitis picture, with superficial enhancement. Look at the available tissue. Does it stink? Think anaerobic bacteria. Is it pus? Think abscess. Is it a nice smooth lump of tissue with a cystlike structure? In the right setting, the gross diagnosis becomes cysticercosis. Finally, examine the smear. Is the tissue normal brain or does it only contain reactive gliosis? It may be near the lesion. If the sample is densely cellular, what constitutes the cellularity? Atypical glia might suggest a glioma but progressive multifocal leukoencephalopathy (PML) should be in the differential. Most inflammatory brain diseases induce some atypia in astrocytes.
The presence of many inflammatory cells is a hallmark of most infectious diseases. A sea of polymorphonuclear leukocytes strongly suggests a bacterial infection. Think meningitis or pyogenic abscess. Multinucleated giant cells and plenty of lymphocytes but no polymorphonuclear lymphocytes (polys) could indicate tuberculosis or sarcoidosis. Adding some polys and active tissue necrosis suggests a fungal infection, Herpes encephalitis, or parasites. Some infectious diseases, notably PML, often invoke little or no inflammatory response in a defective host immune system.
In most instances, an intraoperative evaluation of infected tissue can only suggest that diagnostic tissue is present: you cannot know it is diagnostic unless you can identify the organism. Finding the organism often
requires special stains (e.g., tissue gram stain for bacteria) or immense amounts of microscope time (e.g., toxoplasmosis); both of these are impractical in most intraoperative settings.
requires special stains (e.g., tissue gram stain for bacteria) or immense amounts of microscope time (e.g., toxoplasmosis); both of these are impractical in most intraoperative settings.
Abscesses
Abscesses are easy to identify and you never want to miss them. These lesions can be clinically obvious, for example, in a febrile, sick-looking patient with a recent history of trauma who presents with a focal neurologic deficit and has a ring-enhancing mass on imaging. However, only atypical cases would come to biopsy, such as a healthy-looking afeb-rile patient with no recent surgical procedure who has a similar presentation.
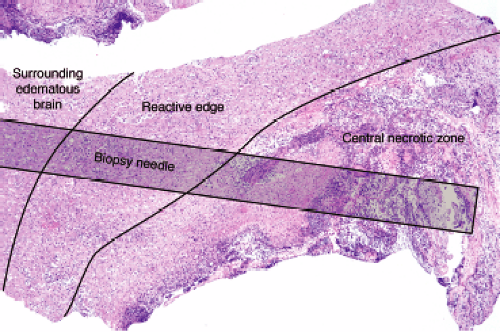
A well-established abscess, the type most likely to undergo biopsy, has three zones that merge at their borders: a central zone of necrotic debris, a highly reactive and organizing edge, and a penumbra of edematous, gliotic brain. The central zone is basically pus and necrotic brain. At the edge of this nonviable tissue, the brain is engaged in a furious effort to wall off the inciting agent and repair the damage. Cells infected with bacteria and bacterial components themselves stimulate host inflammatory cells to release cytokines. These in turn activate microglia, macrophages, and astrocytes; recruit neutrophils and more macrophages; and promote neovascularization. Gradually moving away from the wall, these reactive features dissipate. Surrounding brain typically still has many inflammatory cells and reactive astrocytes, but lacks the newly formed or recently damaged vessels of the wall.
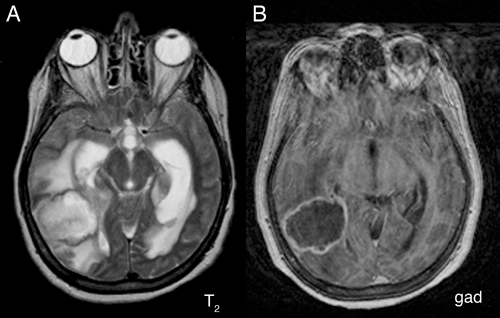
A magnetic resonance imaging (MRI) scan of an abscess exhibits the three zones. The central necrotic region is dead, contains no vessels, and hence does not enhance (Figure 5-1B). It does contain more fluid than normal brain and consequently is brighter on T2-weighted
images (Figure 5-1A). At the still-viable border, leaky new and injured older vessels allow contrast leakage, thus producing the classic sharp rim-enhancement of an abscess (Figure 5-1B). Inflammation that has spilled into the surrounding brain incites cerebral edema and produces the extensive T2-hyperintensity in the adjacent parenchyma (Figure 5-1A). This edema can be much more extensive than is typical of intrinsic neoplastic processes.
images (Figure 5-1A). At the still-viable border, leaky new and injured older vessels allow contrast leakage, thus producing the classic sharp rim-enhancement of an abscess (Figure 5-1B). Inflammation that has spilled into the surrounding brain incites cerebral edema and produces the extensive T2-hyperintensity in the adjacent parenchyma (Figure 5-1A). This edema can be much more extensive than is typical of intrinsic neoplastic processes.
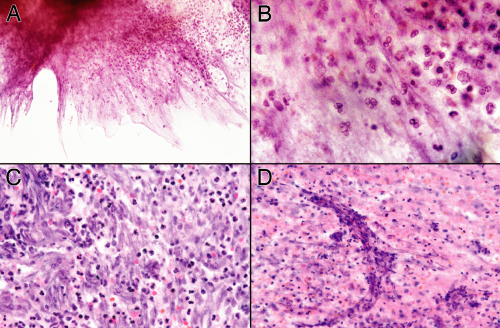
A needle biopsy of a brain abscess can sample one or more of these zones (Figure 5-2). In a typical pyogenic abscess, all three zones will contain neutrophils; these are the key to the diagnosis. Close examination of the tissue at the time of surgery can reveal these regions, although the center area will probably be gooey slime. The edematous, surrounding brain will also have chronic inflammatory cells and reactive astrocytes, because established abscesses form over days to several weeks. The most reactive wall can have extensive neovascularization and abundant mixed but predominantly acute inflammation. The center is pus. All areas should be sampled.
Exactly what the tissue will show depends on how rapidly the abscess has formed and how successfully the body has contained the damage. In its earliest stages, the nascent abscess will look like an acute cerebritis: neutrophils swarming into severely injured or necrotic brain. The wall will not yet exist. As the body fights the organisms at the viable edge, it will show progressive changes from the first neutrophils to activated microglia and later to macrophage infiltration, neovascularization, and more extensive gliosis.
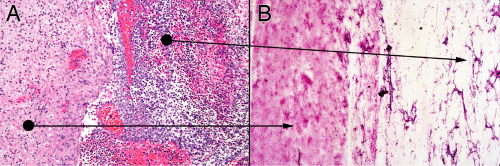
In the smear of early stages, the wall will have neutrophils and some reactive astrocytes. It may not be very cellular (Figure 5-3A). The corresponding smear will also be gliotic, contain some neutrophils, and be relatively hypocellular (Figure 5-3B). Polymorphonuclear leukocytes in a gelatinous, myxoid soup fill the center of the abscess (Figure 5-3B, right side). The smear pulls this mixture apart, leaving stringy collections of dead cells intermixed with polys.
Later in the process, macrophages infiltrate in abundance. These cells release many of the important cytokines
that help organize necrotic brain tissue. In the smear, the edge will become hypercellular, gliotic, and contain many macrophages and neutrophils (Figure 5-4).
that help organize necrotic brain tissue. In the smear, the edge will become hypercellular, gliotic, and contain many macrophages and neutrophils (Figure 5-4).
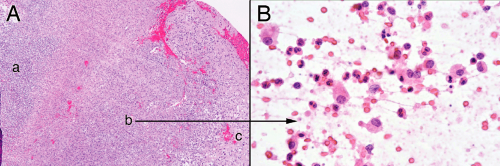
The necrosis in the center of an abscess derives from bacteria directly killing cells, ischemia from destroyed vessels, and bystander effects of the lysosomal killing mechanisms (e.g., reactive oxygen species). The dead cells release their proteins and nucleic acids into the matrix, which then mixes with the remaining neutrophils. Rather than coagulative necrosis, this process produces a liquifactive necrosis. In a smear, the necrotic debris tied up with cellular and matrix elements spreads out as jagged myxoid fingers or strings embedded with neutrophils (Figure 5-5A). Polys are the only recognizable cell type (Figure 5-5B). The process of preparing a smear commonly stretches out the dead nuclei and leaves nuclear streaks (Figure 5-5C). As the center zone “matures,” all of the material becomes necrotic. Broken-down nuclei or karyorrhectic debris from neutrophils, washed out and homogenously basophilic nuclear ghosts, streaks of broken-down nuclei, and other unrecognizable material comprise the necrotic debris on the smear (Figure 5-5D). The necrosis within an abscess differs from regular brain necrosis by the preponderance of neutrophils in the former and the dominance of macrophages in the latter.
Toxoplasmosis
Most of us who have been exposed to cats probably have been infected with Toxoplasma gondii. Cats, the definitive
host of these parasites, shed the organism in their feces. Watching the dust storms created by a cat in its litterbox leaves little doubt about how the parasite could be transmitted. For most of us, an infection is inconsequential, perhaps an enlarged lymph node. Only in an immunocompromised host or a fetus does a T. gondii infection have consequences. Brain biopsies that turn out to be toxoplasmosis come from AIDS patients or other immunologically incompetent hosts.
host of these parasites, shed the organism in their feces. Watching the dust storms created by a cat in its litterbox leaves little doubt about how the parasite could be transmitted. For most of us, an infection is inconsequential, perhaps an enlarged lymph node. Only in an immunocompromised host or a fetus does a T. gondii infection have consequences. Brain biopsies that turn out to be toxoplasmosis come from AIDS patients or other immunologically incompetent hosts.
T. gondii is an obligate intracellular parasite, in the same phylum as malaria. In the brain of an immunocompromised host, it can infect both glia and neurons. These host cells become bloated with parasites and form diagnostic “bradyzoites.” When the cell finally ruptures, these parasites spill out into the surrounding milieu as single organisms, ready to infect the next cell. Isolated parasites move more quickly and are thus termed tachyzoites




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree