Metastatic tumors occur anywhere along the neural axis and in any of the main compartments of the head (e.g., intradural and extramedullary). They are the most common brain tumor, increase in frequency with age, and will show an ever-increasing incidence as our population grays. Smears are accurate in diagnosing most brain metastases.
By the time they reach the central nervous system, the majority of metastatic tumors are growing rapidly. Patients usually have only a short history of seizures, pain, or a focal neurologic deficit. Some tumor types, notably lung and kidney cancer, often present clinically as metastases to the brain. In such cases, the intraoperative consultation initiates or augments the search for the unknown primary. When faced with a metastasis, the two most important aspects of the clinical history are: does the patient already have a known primary, and does the patient smoke? The pathologist must always bear in mind, however, that even patients with one primary are not immune from developing a second tumor. This is especially true of breast cancer, which is both common and in many cases treatable. All neuropathologists remember patients with a history of breast cancer who develop either a benign meningioma or a malignant glioma that is only recognized during the intraoperative consultation.
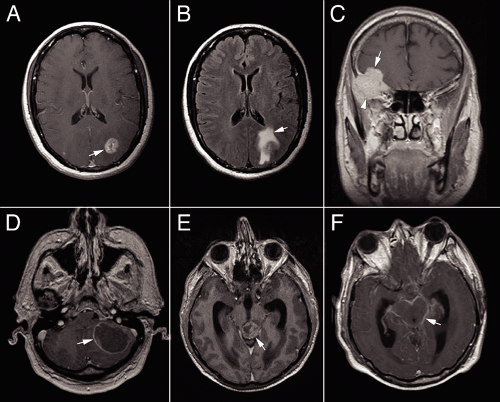
In the normal physiologic state, the blood–brain barrier excludes contrast agents. Metastatic tumor neovascularization fails to recapitulate the endothelial cell tight junctions of this barrier; hence, metastases enhance in neuroimaging. The patterns of enhancement, as well as the effects on the surrounding brain, give clues as to the nature of a brain tumor. Unlike gliomas, metastatic tumors grow as alien invaders within brain parenchyma, pushing away and destroying its host rather than infiltrating among its cells. In neuroimaging, such foreigners typically develop a sharp border of enhancement (Figure 12-1). Internally, metastases often outgrow their vascular supply and become necrotic, thus giving a heterogeneous or ring pattern of enhancement. This is especially true of lung and breast metastases. Other tumors, such as renal cell carcinomas, occasionally grow more slowly and keep pace with their neovascularization; such neoplasms frequently uniformly enhance. Metastatic tumors induce reactive changes in the adjacent brain, including gliosis and edema. Water retained in edematous tissue increases the T2 and FLAIR signals on magnetic resonance imaging scans (Figure 12-1B) and creates corresponding hypodense signals on CT. Even small metastases can generate extensive FLAIR signal. Metastatic tumor should be suspected in any enhancing mass with a sharp, 360-degree border that also induces surrounding FLAIR or T2 signal. Multiple masses also support this diagnosis; however, beware of “multifocal glioblastoma” and primary lymphoma (see Figures 6-17 and 9-8).
The most common tumors to metastasize to brain include adenocarcinomas (especially from the lung and breast), small cell or neuroendocrine-type carcinomas, renal cell carcinoma, and melanoma. Their cytology will be discussed subsequently. However, almost any metastasizing tumor can reach the brain. Unlike most gastrointestinal tumors, rectal carcinomas can metastasize to brain without first presenting in the liver. Choriocarcinoma and papillary thyroid carcinoma, along with the more common lung cancers and melanomas, often present as hemorrhages; the blood usually contains a few diagnostic cells. Prostate cancer has three notable features: it does not enter into the brain, it has a predilection for the dura, and it can be cytologically relatively bland; all of these features are shared with meningiomas.
A frequent and legitimate question is, “Why bother to resect a metastasis?” Why crack open someone’s skull, with all of its attendant morbidity, and remove a mass, when an unseen cluster of tumor cells could lurk
nearby? In some locations, such as in the posterior fossa or large supratentorial tumors, resection postpones impending death. Metastases produce mass effect by their size and by the edema they generate. Unchecked, this effect can rapidly lead to herniation and death. Several studies show improved patient survival if a single brain metastasis is removed. Finally, when faced with a relatively young, otherwise healthy patient who has a brain metastasis, heroic measures seem morally, if not medically, justified.
nearby? In some locations, such as in the posterior fossa or large supratentorial tumors, resection postpones impending death. Metastases produce mass effect by their size and by the edema they generate. Unchecked, this effect can rapidly lead to herniation and death. Several studies show improved patient survival if a single brain metastasis is removed. Finally, when faced with a relatively young, otherwise healthy patient who has a brain metastasis, heroic measures seem morally, if not medically, justified.
Anatomic pathologists also wonder, “Why bother to smear a resected metastasis?” Tissue is often abundant and a frozen section usually distinguishes such tumors from those originating within the brain. Aside from their speed and accuracy, smears provide additional structural information about a tumor that may be helpful in
difficult cases. A frozen section never augments and can, by its artifacts and tissue destruction, hinder a final diagnosis. Smears always provide a different and complementary type of biophysical information that often augments or reinforces a final diagnosis. A smear also provides cytological detail that is frequently obscured in the final histology. Salt-and-pepper chromatin never is clearer than in a fresh cytological preparation. In a patient with a known primary tumor, the intraoperative consultation confirms the diagnosis of a metastasis and excludes abscesses or possibly a second tumor; the smear excels at both. In cases of radiologically uncertain tumors in patients without a known primary, the consultation places the tumor in a major category (e.g., glioma, metastasis) and allows an appropriate pathological workup to begin earlier; the smear usually provides the necessary information and adds structural details for the final diagnosis.
difficult cases. A frozen section never augments and can, by its artifacts and tissue destruction, hinder a final diagnosis. Smears always provide a different and complementary type of biophysical information that often augments or reinforces a final diagnosis. A smear also provides cytological detail that is frequently obscured in the final histology. Salt-and-pepper chromatin never is clearer than in a fresh cytological preparation. In a patient with a known primary tumor, the intraoperative consultation confirms the diagnosis of a metastasis and excludes abscesses or possibly a second tumor; the smear excels at both. In cases of radiologically uncertain tumors in patients without a known primary, the consultation places the tumor in a major category (e.g., glioma, metastasis) and allows an appropriate pathological workup to begin earlier; the smear usually provides the necessary information and adds structural details for the final diagnosis.
Malignant Melanomas
Malignant melanomas span a greater range of patient ages than most other metastatic tumors. This neoplasm afflicts both young and aged adults. By the time they reach the brain, many melanomas will have presented elsewhere in the body. Like breast cancer but unlike most other carcinomas, these neural crest tumors can develop within nervous system tissue many years after their primaries were thought cured. Melanomas belong to the group of hemorrhagic tumors; their resected masses occasionally consist almost entirely of blood and blood-breakdown products, with only a few diagnostic cells remaining. Hemosiderin-laden and activated macrophages look deceptively like melanoma cells. On neuroimaging, melanomas can uniformly enhance, ring-enhance, or be buried in a sea of blood.
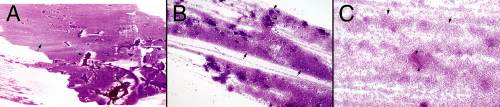
A significant subset of melanomas continues to produce pigment, even after they metastasize. They are almost diagnosed on gross inspection by their lamp-black color. Uncertainty arises from trying to distinguish black from the dark brown color of old hemorrhage. Of the several types of melanoma, those that reach the central nervous system usually show little cell–cell affinity. These discohesive tumors typically smear evenly across the glass. At low magnification or even when holding the slide up to the light, the neoplasm forms a smooth cellular gradient (Figure 12-2). Other tumors giving similar low-power gradients of discohesive cells include lymphomas, adenomas, and neurocytomas; all of these are easily distinguished from melanoma at higher powers. It is this low-power cellular gradient, when combined with the high-power cytologic features, that makes the smear so useful in diagnosing these tumors.
At intermediate microscope powers, melanomas elaborate no significant matrix (Figure 12-3). Their cells float on the glass slide, unfettered by any strands or processes. Like all diseases that injure the brain, these tumors induce secondary gliosis, including some “atypical” astrocytes. The reactive glial cells, tethered to their matrix, can be “atypical” but remain heterogeneous and do not display the anaplasia of their unwelcome visitors.
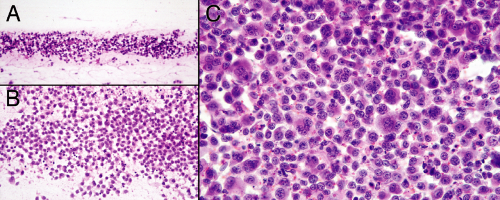
Cytologically, melanomas range from monotonous (Figure 12-4) to wildly pleomorphic. Binucleated and multinucleated forms often pepper microscopic fields. The classic melanoma cytological feature of a central
prominent nucleolus is not always obvious or even present. Cells from different tumors do, however, share two common characteristics: nuclei are anaplastic and cytoplasm is plentiful. Melanoma nuclei are large, have coarse chromatin, and often have a prominent nucleolus. Unlike high-grade gliomas, lymphomas, and many carcinomas, their nuclear membranes are not necessarily convoluted, irregular, or folded (Figure 12-4, A and B). Compared to many other high-grade tumors showing similar noncohesive properties (e.g., lymphomas and signet-ring cell adenocarcinomas), melanoma cells typically have abundant cytoplasm that remains sharply demarcated (Figure 12-4, arrowheads (B and C) and arrows (E and F)). Only the most poorly differentiated melanomas lose their cytoplasm. Several ancillary features support the diagnosis of melanoma. Occasional or sometimes frequent cells within a tumor contain diagnostic fine melanin pigment in their cytoplasm (Figure 12-4A, arrow). Even a few such cells under the microscope usually render these tumors lamp-black to the eye. However, finding fine pigment outside of tumor cells (Figure 12-4A, arrowhead), although suggestive of melanin, should not be considered diagnostic. A subpopulation of melanoma cells may contain intranuclear inclusions (Figure 12-4C). Some cells also have cytoplasmic vacuoles (Figure 12-4B, arrow); however, these are more likely to confuse the tumor with an adenocarcinoma rather than augment the diagnosis.
prominent nucleolus is not always obvious or even present. Cells from different tumors do, however, share two common characteristics: nuclei are anaplastic and cytoplasm is plentiful. Melanoma nuclei are large, have coarse chromatin, and often have a prominent nucleolus. Unlike high-grade gliomas, lymphomas, and many carcinomas, their nuclear membranes are not necessarily convoluted, irregular, or folded (Figure 12-4, A and B). Compared to many other high-grade tumors showing similar noncohesive properties (e.g., lymphomas and signet-ring cell adenocarcinomas), melanoma cells typically have abundant cytoplasm that remains sharply demarcated (Figure 12-4, arrowheads (B and C) and arrows (E and F)). Only the most poorly differentiated melanomas lose their cytoplasm. Several ancillary features support the diagnosis of melanoma. Occasional or sometimes frequent cells within a tumor contain diagnostic fine melanin pigment in their cytoplasm (Figure 12-4A, arrow). Even a few such cells under the microscope usually render these tumors lamp-black to the eye. However, finding fine pigment outside of tumor cells (Figure 12-4A, arrowhead), although suggestive of melanin, should not be considered diagnostic. A subpopulation of melanoma cells may contain intranuclear inclusions (Figure 12-4C). Some cells also have cytoplasmic vacuoles (Figure 12-4B, arrow); however, these are more likely to confuse the tumor with an adenocarcinoma rather than augment the diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree