Introduction
Non-neoplastic brain diseases are particularly difficult to the pathologist for two reasons: they are rare and many of their pathologic changes are nonspecific. In well-characterized diseases, such as multiple sclerosis, biopsies are rarely indicated. Such lesions undergo biopsy only when they are unsuspected clinically or because they are atypical. Nearly all brain lesions contain reactive gliosis and many have some form of inflammation. Unlike common brain tumors, most pathologists never gain enough experience to feel comfortable examining such cases. Intraoperative examination of such lesions only compounds the difficulties. A frozen section from the center of a demyelinating lesion will look cellular, glial, and contain some atypical glial cells; these are all features of a glioma. Smears are also not a panacea but can provide important clues that the lesion is not neoplastic.
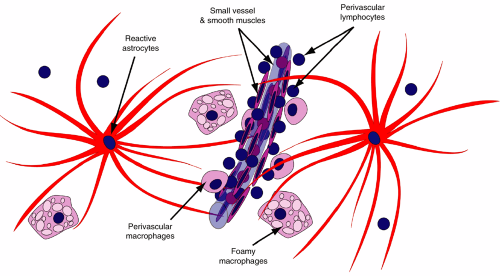
Reactive brain lesions characteristically have a mixed population of cells, including astrocytes, macrophages, and lymphocytes (Figure 4-1). Like a royal family’s elite guard, astrocytes react to most brain injuries, enclosing and protecting their neuronal and axonal charges. Ischemia, inflammation, trauma, radiation, and neoplasia of any sort induce reactive astroglial changes. Early in this process, astrocytes pour out copious quantities of intermediate filaments (e.g., glial fibrillary acidic protein [GFAP]). These proteins typically accumulate near the nucleus soon after an injury and later move out to the distal radiations of the astrocytes. Unlike other organs in the body, the brain does not usually use the fibroblast to form scars and no regular scar contraction occurs in brain lesions. Instead, a meshwork of delicate astrocyte fibers wall-off injuries and form brain “scars.” The sanitation engineers of the brain are its microglia and their peripheral brethren, the macrophages. While astrocytes support remaining neurons and axons, macrophages clean up the debris left by a destructive lesion. In many cases, brain injuries also induce some level of inflammation. Signals from acute destruction recruit neutrophils. Subacute or chronic lesions signal lymphocytes to extravasate into the brain. These lymphocytes congregate first around vessels (both T- and B-cells) and, in immune-mediated lesions such as multiple sclerosis or viral infections, infiltrate brain parenchyma (mainly T-cells).
Reactive Astrocytosis
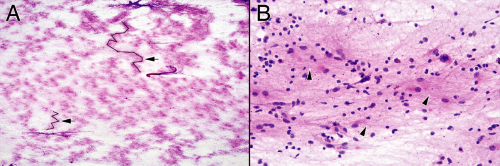
At very low magnification, smears from reactive brain injuries display a “fluffiness” characteristic of gliosis (Figure 4-2A). Local astrocyte nuclei and other cells embedded in their gliotic meshwork remain bound together, whereas more distant groups are sheared away in their own clusters. Fibers peripheral to the groups bridge other cellular collections together. This fluffy pattern of sheared nuclei and their filaments is akin to cotton pulled apart with its cottonseeds. Usually, reactions can be distinguished from neoplasia by the low-magnification cellularity of the glial islands; reactions typically do not achieve the cellular density characteristic of a neoplastic process. Inflammation adds to the cellularity but purely reactive glial clusters are hypocellular and their nuclei are small.
Intermediate magnifications begin to resolve the glial background. The fuzzy bridges and halos around the clusters break apart into fine astrocytic strands. Some strands clearly originate from thicker processes around astrocytic nuclei, whereas others seem to wander aimlessly among the other cells (Figure 4-2B). These magnifications reveal the cellular heterogeneity of a reactive process. Larger reactive astrocytic nuclei swim in the same lake with small cells (oligodendrocytes and possibly
lymphocytes), fusiform cells (microglia), and perhaps a foamy macrophage.
lymphocytes), fusiform cells (microglia), and perhaps a foamy macrophage.
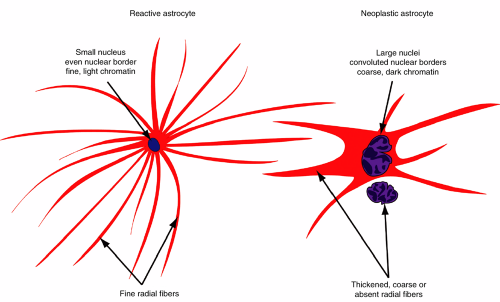
Confirming the reactive glial character of the process requires high magnification (Figure 4-3). A reactive astrocyte typically has a small, benign nucleus that lacks the coarse chromatin, hyperchromasia, or convoluted nuclear membrane that typifies a neoplastic astrocyte (Figure 4-4). More telling are its fine astroglial processes. These reactive cells are programmed to produce many fine radiations, each filled with intermediate filaments. The filaments are synthesized near the nucleus then transported to the distal and delicate radiations.
When plump with perinuclear intermediate filaments, the cells are termed gemistocytes. Neoplastic astrocytes gradually lose this ability to form fine distal processes in a manner proportional to their loss of differentiation and inversely proportional with their increasing anaplasia (Figure 4-4).
When plump with perinuclear intermediate filaments, the cells are termed gemistocytes. Neoplastic astrocytes gradually lose this ability to form fine distal processes in a manner proportional to their loss of differentiation and inversely proportional with their increasing anaplasia (Figure 4-4).
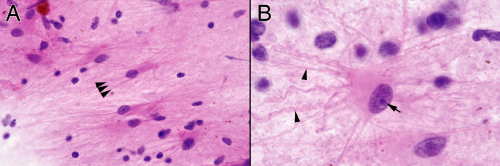 FIGURE 4-3. Higher power views of Figure 4-2. A. Fine, eosinophilic processes radiating off an astrocyte. Notice the size variation in the nuclei; reactive processes contain a mixture of cell types and hence typically have a heterogeneous appearance. B. High magnification showing a fully developed reactive astrocyte that spawned many fine and some coarser glial fibers (arrowheads) from its usually eccentric, plump cytoplasm. Its nucleus has a smooth border, relatively fine chromatin, and a prominent nucleolus (arrow). In this biopsy near a hemorrhagic melanoma, the astrocytes have taken up a small amount of pigment. Such cells reside around any type of brain hemorrhage. The plump perinuclear material indicates the cell was actively producing intermediate filaments and slowly transporting them to the outer reaches of the astrocytic radiations. |
Because nearly every injury to the central nervous system induces gliosis and many diseases infiltrate the parenchyma, evil often lurks in a reactive picture. Primary central nervous system lymphomas notoriously can permeate the brain’s substance and produce a reactive smear (Figure 4-5). However, even in highly infiltrative cases, the smear differs from a purely reactive pattern. It will be more cellular and will hide the neoplastic cells within the fine glial matrix. A low-power examination of a smear will demonstrate the glial nature of a lesion; its details or etiology are found at high power.
Inflammation
Like the rest of the body, inflammation can both protect and injure the brain. In acute bacterial meningitis, the flood of neutrophils into the subarachnoid space clearly demonstrates the body’s attempt to destroy the invaders, whereas in the active demyelination of multiple sclerosis, the white matter T-lymphocytes lack a known inciting pathogen. In both intraoperative smears and permanent sections, the type of inflammation provides clues to the underlying etiology of a lesion. Polymorphonuclear leukocytes suggest two possibilities: acute tissue destruction or acute bacterial infection. A mixed inflammatory infiltrate of polymorphonuclear leukocytes (polys) and lymphocytes hints at a lesion that is both actively destroying tissue and has been around for a while. For example, fungal infections and vasculitis can have such mixed infiltrates. Aside from indicating chronicity, a predominantly lymphocytic infiltrate is less specific. A wide variety of stimuli recruit these immune cells, including irritants (e.g., foreign bodies), many viral infections, and self-antigens in primary inflammatory diseases. When tissue destruction involves brain parenchyma, the macrophage rather than the neutrophil is the primary cell enlisted to remove the dead tissue. Macrophages remove necrotic debris from infarcts, other necrotizing injuries such as trauma or infections, myelin debris in multiple sclerosis, and regressing nervous system elements such as in Wallerian degeneration. The brain has its own resident macrophages, the microglia, which are among the first cells recruited to injured brain tissue.
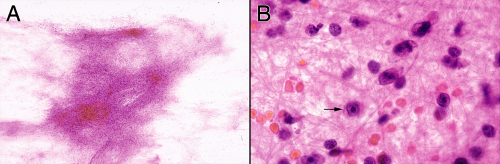 FIGURE 4-5. Smear prepared from a stereotactic biopsy of a primary central nervous system lymphoma. At low power (A), the smear has a glial quality, including its fluffiness and fibrillary borders. However, even at this power, the tissue is much more cellular than a purely reactive process (compare with Figure 4-3). Only at high magnification (B) can the neoplastic cells be identified (arrow), hiding in the fine glial matrix. Other fields of the smear had more diagnostic cells within a less dense glial matrix. |
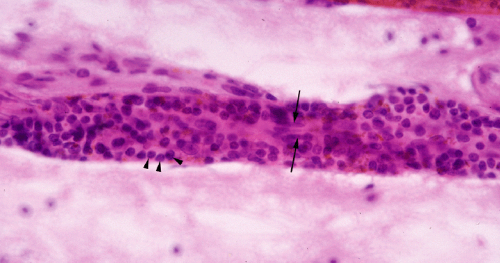
Inflammatory cells typically shed easily in a smear, so recognizing them and understanding what they indicate will provide clues to the diagnosis. However, recognizing the cell type might not be easy. Lymphocytes alone, especially infiltrating T-cells, may be difficult to distinguish from oligodendrocytes or small, granular neurons. On either a smear or in permanent hematoxylin and eosin (H&E) sections, one clue is that lymphocytes naturally congregate around small vessels (Figure 4-6). Neutrophils are usually easy to identify; just look for their nuclei with three or four subdivisions (see abscess below).
In contrast to polys and lymphocytes, macrophages notoriously hide their identity. These phagocytic cells bulge with debris-containing vacuoles, which render their membranes fragile and easily disrupted during a smear. Frozen sections are usually even less reliable, because the cytoplasmic phagocytic vacuoles can be unapparent or difficult to distinguish from artifacts. However, macro-phages are extremely important to recognize because their presence indicates necrosis or degeneration. Macro-phages signify a reaction to injury, with all of its attendant secondary and sometimes atypical changes in astrocytes; they should always make you second-guess your initial diagnosis of neoplasia. On a smear, these cells can remain intact in thicker regions, where surrounding cells protect their membranes. Typically they have abundant, foamy cytoplasm, distinct cytoplasmic borders, and round, eccentrically placed nuclei (Figure 4-7A




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree