Dr. Elizabeth Bundock MD, PhD
Written in conjunction with
The tumors discussed in this chapter range from essentially malformations to the most malignant of brain tumors. They have in common an expression of some neural differentiation. Anyone who has taken a basic neuroscience course knows what a neuron is: a cell designed to receive myriad of electrochemical inputs, integrate them, translate them into frequency-encoded electrical signals, and then transmit them long distances to other neurons or muscle. However, none of this information is useful in the diagnosis of neural tumors. Neurons take on a large range of morphologies and sizes, from the great Betz motor neurons in the precentral gyrus to the lowly granular neuron of the cerebellum. In many cases, no single morphological feature will define a cell as neuronal. For many neuronal tumors, the diagnosis is made on several grounds, including cytological features, immunophenotype, and possibly electron microscopy.
A large cell having bluish, granular material in the cytoplasm and a large nucleus with a single, prominent nucleolus and fine chromatin can easily be identified as a “ganglion cell.” Finding such cells in a tumor raises the possibility of either a “gangliocytoma” or a “ganglioglioma.” The former borders on a malformation or hamartoma and consists mostly of easily identified ganglion cells. The latter has other cellular elements, including glial cells and some cells having an intermediate differentiation. In this book, these two sometimes arbitrarily distinguished tumors will be combined into “ganglion cell tumors.” When the neuronal elements resemble mature granular neurons, rather than pyramidal or ganglion neurons, cytological features alone become unreliable. These granular-type neural tumors contain cells having scant cytoplasm and their nuclei typically lack a large, dominant nucleolus. What gives them away as neuronal is the company they keep: they tend to be extremely monotonous and produce a fine neuropil matrix. This matrix incorporates many synaptic proteins, including synaptophysin, thus giving another method to identify them. The malignant spectrum of neural tumors derives mainly from primitive neural precursors, especially from the cerebellar external granular neuroblasts. Morphologic features of various “blastomas” (i.e., neuroblastoma and medulloblastoma) range from very primitive cells displaying minimal neural features to those having significant neuronal differentiation, including production of abundant neuropil.
Many of the neural tumors have a penchant for growth near the outer border of the brain. Frequently they grow into the subarachnoid space. This, by itself, is not a malignant feature, even though such spread should be mentioned in a pathology report. Subarachnoid growth, unfettered by normal biological constraints, often elicits a distinctly different pattern of growth than that within brain parenchyma. Inflammatory responses and desmoplasia frequently accompany such tumors. Distinct from subarachnoid growth, the primitive neural tumors, including medulloblastoma, can “seed” the cerebrospinal fluid and spread to other levels of the nervous system. These truly malignant tumors require treating the entire craniospinal axis, rather than just the tumor bed.
Ganglion Cell Tumors
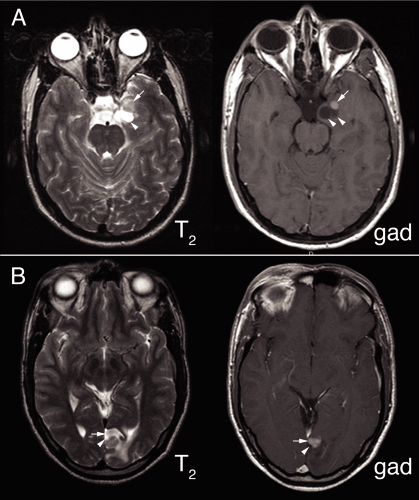
Neuroectodermal tumors containing mature-looking neurons or “ganglion cells” predominantly present in children and young adults as focal seizures. These patients may have a long history of epilepsy, although perhaps less so than in the past due to the ready availability of magnetic resonance imaging (MRI). The tumors can occur anywhere along the neural axis, including the cerebellum and spinal
cord; however, they most commonly arise in the cerebral cortex, especially in the temporal lobes. Unlike normal brain, the vessels supplying these tumors leak; hence they enhance in neuroimaging. They typically have a nonenhancing cystic component with an enhancing nodule or nodules on one side (Figure 8-1).
cord; however, they most commonly arise in the cerebral cortex, especially in the temporal lobes. Unlike normal brain, the vessels supplying these tumors leak; hence they enhance in neuroimaging. They typically have a nonenhancing cystic component with an enhancing nodule or nodules on one side (Figure 8-1).
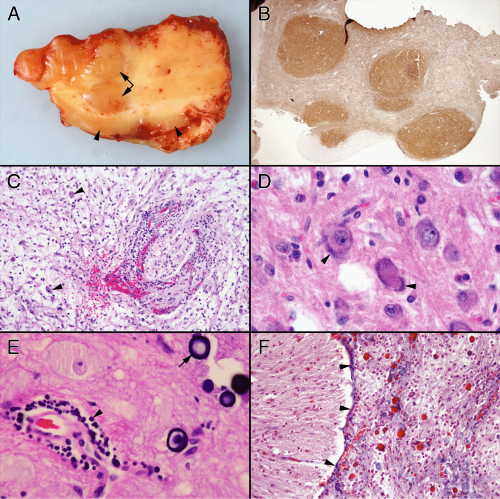
Observing what these tumors look like in routine examination helps explain their appearance in intraoperative smears (Figure 8-2). Tumors containing neurons express variable amounts of neuropil, which to some extent makes them resemble gray matter. They often evoke a chronic inflammatory response. In the brain, these reactions predominate around vessels but also extend into the adjacent tumor or brain. At high magnification, they usually contain several cell types but, by definition, need to contain ganglion-like cells. Unlike normal neurons, these cells tend to have distorted features, including bizarre shapes with several oddly oriented processes, patchy or peripherally marginated but thickened Nissl substance, anomalous tight clustering of large neurons, or binucleated forms. Other cell types include atypical astroglial cells and smaller, neurocytoma or granular neuron forms. That these tumors typically grow slowly and cause reactive changes in their host tissue is reflected in their penchant to form mineralized deposits. Such “calcifications” form around vessels or in the tissue. Although malignant tumors often spread freely into the subarachnoid space, such growth in primary brain tumors does not necessarily reflect a high grade. Pilocytic astrocytomas and gangliogliomas frequently fill the meningeal space above their primary tumor. Within this topological space, the tumors become subject to systemic tissue reactions, in addition to the normal reactions in the brain. They often induce considerable inflammation, fibrosis, collagen deposition, and reticulin deposition, all of which can make a smear more cohesive and difficult to interpret.
What ganglion cell tumors look like in a smear depends in part on their glial components and in part on their subarachnoid spread. The power of the smear in these cases lies in your eye’s ability to discern subtle changes in the gross findings. Sampling each distinct zone will produce a more informative smear, increase the accuracy of the intraoperative diagnosis, and allow you to better use the structural information in the smear during the final sign-out of the permanent sections.
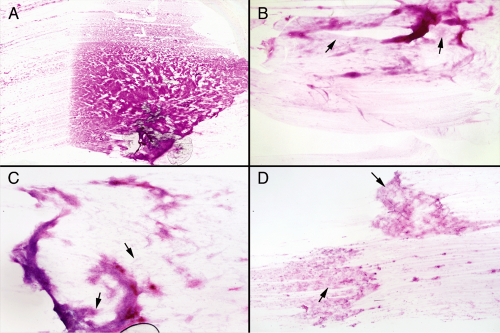
At a low magnification, these tumors may exhibit the cottony appearance of a glial tumor, a more cohesive appearance due to induced inflammation and fibrosis, or looser, gossamer neoplasm befitting of neurons (Figure 8-3). A microscope slide of a smear, laid on a white piece of paper, often has a bluish hue around the tissue due to the tumor’s increased glycosaminoglycan matrix. Within this light matrix, tumor cells spread relatively easily. The most informative areas in such smears are at the edges of the denser zones, where the cells spread out easily into a monolayer (Figure 8-3).
Intermediate microscopic powers (Figure 8-4) demonstrate vessel structure, matrix elements, and the degree of cellular heterogeneity. Like antlers on a deer, many vessels will spread out in the smear in delicate, small branches and capillaries. These are not the coarsely thickened vessels surrounded by dense astroglial processes of a high-grade glioma, but instead are reminiscent of vessels found in normal brain. Gangliogliomas frequently induce a significant inflammatory reaction with predilection for small and intermediate size vessels. On the smear, look for thickened vessels having a dense infiltrate of small, round blue cells. The presence of inflammation imparts a distinct cellular heterogeneity to the tumors, with cells ranging in size from small lymphocytes to the large neuronal components. The tumor matrix expresses variable degrees of gliosis; however, the processes are usually fine, not thick and coarsened, which makes them look more like smoke than cotton. Some tumors elaborate few if any glial elements. Often, the matrix background will be soft and slightly basophilic. Although producing a form of neuropil, the tumor matrix lacks the uniformity and eosinophilia of normal gray matter. The key to the diagnosis of a mature neuronal tumor is finding a population of larger cells spread out in the finer matrix zones. Occasionally, these jump out of the slide at low power; more typically such cells lie buried in a larger sea of mixed cellularity, including inflammation but also other granular or neurocytoma-like neuronal elements. Search for the subpopulation of large cells. A diagnostic dilemma arises in trying to differentiate a ganglion cell tumor from an astrocytoma infiltrating gray matter. Distinguishing neoplastic or dysplastic ganglion cells from normal neurons surrounded by tumor usually requires high magnification. However, the matrices of these two tumors frequently differ: neuronal tumors often elaborate a loose myxoid background, whereas glial tumors produce a denser glial architecture. Neuron-appearing cells in a basophilic background suggest a ganglion cell tumor; in a dense glial background, they suggest a glioma infiltrating gray matter.
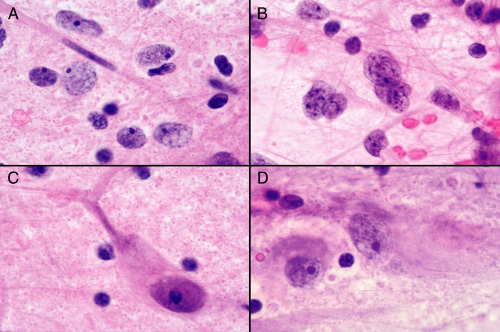
Finding diagnostic “ganglion cells” or cells of unmistakable neuronal character is key to the diagnosis. As mentioned previously, these cells have a large nucleus with a large nucleolus and relatively fine or salt-and-pepper chromatin. The shearing forces of the smear and the minimal structural integrity of the large cells often
combine to strip the nuclei of their cytoplasm (Figure 8-5A). The size of these cells, the absence of anaplastic features (e.g., complex, folded nuclear membranes, coarse chromatin), and their prominent nucleolus leaves little doubt about their phenotype. Usually some intact cells remain, often in protected regions of the smear (e.g., braced by vessels or in thicker regions of the smear). Such cells (Figure 8-5, C and D) need to be distinguished from normal cortical neurons. The latter typically have a well-defined pyramidal structure, whereas the former often have abnormally distributed Nissl substance; thick, haphazardly arranged cell processes; or have odd distributions or clustering adjacent to each other. In addition to ganglion cells, gangliogliomas have a distinct, neoplastic glial element (Figure 8-5B). These cells are more anaplastic than their neuronal counterparts and have glial processes attached to their nuclei. Alone they could be worrisome for a high-grade glioma; the neighbors they keep and the neighborhood they live in (e.g., ganglion cells, neuropil matrix, hypocellular) distinguish them from more aggressive tumors.
combine to strip the nuclei of their cytoplasm (Figure 8-5A). The size of these cells, the absence of anaplastic features (e.g., complex, folded nuclear membranes, coarse chromatin), and their prominent nucleolus leaves little doubt about their phenotype. Usually some intact cells remain, often in protected regions of the smear (e.g., braced by vessels or in thicker regions of the smear). Such cells (Figure 8-5, C and D) need to be distinguished from normal cortical neurons. The latter typically have a well-defined pyramidal structure, whereas the former often have abnormally distributed Nissl substance; thick, haphazardly arranged cell processes; or have odd distributions or clustering adjacent to each other. In addition to ganglion cells, gangliogliomas have a distinct, neoplastic glial element (Figure 8-5B). These cells are more anaplastic than their neuronal counterparts and have glial processes attached to their nuclei. Alone they could be worrisome for a high-grade glioma; the neighbors they keep and the neighborhood they live in (e.g., ganglion cells, neuropil matrix, hypocellular) distinguish them from more aggressive tumors.
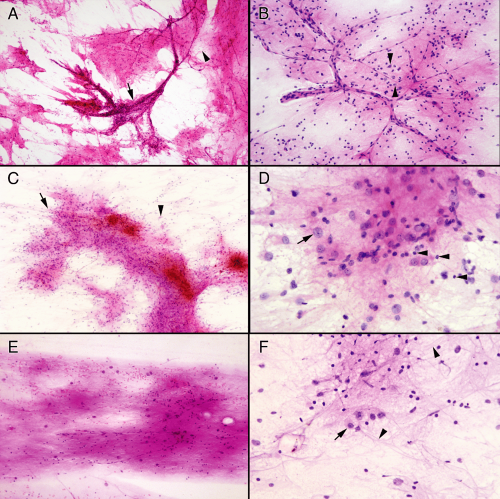
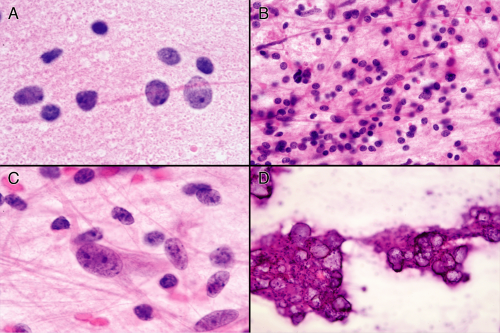
Several ancillary features of these tumors aid in evaluating their smears. Foremost among these is the neuropil they generate. This feature alone should suggest you are looking at either a neuronal tumor or gray matter. Neuropil is not homogenous but instead is a complex mixture of dendrites, axons, and synapses. In the smear, these tubular structures fragment and produce innumerable tiny lightly eosinophilic spheres (Figure 8-6). Best examined by turning down the condenser, these pink granules spread evenly over the slide. Look for this material in the background of the glial matrix or abutting small vessels. Ganglion cell nuclei often seem to float in
this sea of synapses. These tumors elicit chronic reactive changes in the involved tissues. Perivascular chronic inflammation (Figure 8-4A) and tumor inflammation (Figure 8-6B) are common. Away from vessels, dispersed lymphocytes may be difficult to distinguish from either small granular neurons or oligodendrocyte nuclei. Do not look at single cells but rather the population of cells. Remember, except in the cerebellum, granular neurons typically do not overwhelm a smear. Mineralized concretions, usually just called “calcifications,” frequently accompany regions of ganglion cell tumors (Figure 8-6). These deposits encrust vessels or remain separate in the matrix. Many persistent injuries to the brain induce such deposits. Finally, tumors of mixed neuronal and glial origins often have cells of indeterminate phenotype (Figure 8-6C), with features of both glia and neurons. Hypothesizing that a primitive astroglial cell represents the ground state of the central nervous system helps explain how such mixtures of cells develop and why many tumors that dedifferentiate (or “go bad”) show increasing astroglial features.
this sea of synapses. These tumors elicit chronic reactive changes in the involved tissues. Perivascular chronic inflammation (Figure 8-4A) and tumor inflammation (Figure 8-6B) are common. Away from vessels, dispersed lymphocytes may be difficult to distinguish from either small granular neurons or oligodendrocyte nuclei. Do not look at single cells but rather the population of cells. Remember, except in the cerebellum, granular neurons typically do not overwhelm a smear. Mineralized concretions, usually just called “calcifications,” frequently accompany regions of ganglion cell tumors (Figure 8-6). These deposits encrust vessels or remain separate in the matrix. Many persistent injuries to the brain induce such deposits. Finally, tumors of mixed neuronal and glial origins often have cells of indeterminate phenotype (Figure 8-6C), with features of both glia and neurons. Hypothesizing that a primitive astroglial cell represents the ground state of the central nervous system helps explain how such mixtures of cells develop and why many tumors that dedifferentiate (or “go bad”) show increasing astroglial features.
On a smear, the main differential diagnoses of a ganglioglioma include fibrillary gliomas infiltrating cortex and other tumors featuring large mature-looking neurons, especially a dysembryoplastic neuroepithelial tumor (DNT). Fortunately, the latter is not really an issue because the key intraoperative information required by a surgeon is that the tumor has a mature neuronal component and is low grade. Gliomas infiltrating cortex are more problematic because neuropil and neurons will be present. To be diagnostic for a ganglioglioma, a tumor needs to elaborate atypical neurons or just generate too many relatively uniform neurons in a glial-myxoid matrix. When gliomas infiltrate cortex, the normally low population
of large neurons decreases further. Many large or atypical neurons in a smear should suggest a neuronal tumor. Although gangliogliomas have some glial matrix, it should be well differentiated. A thickened matrix containing coarse astroglial processes with a few scattered, mature neurons should suggest an astrocytoma that is invading cortex. Sometimes distinguishing a low-grade, cortex-loving oligodendroglioma from a mature neuron-producing tumor requires permanent sections, special studies, and reevaluation of the intraoperative smear.
of large neurons decreases further. Many large or atypical neurons in a smear should suggest a neuronal tumor. Although gangliogliomas have some glial matrix, it should be well differentiated. A thickened matrix containing coarse astroglial processes with a few scattered, mature neurons should suggest an astrocytoma that is invading cortex. Sometimes distinguishing a low-grade, cortex-loving oligodendroglioma from a mature neuron-producing tumor requires permanent sections, special studies, and reevaluation of the intraoperative smear.
Central Neurocytomas
Central neurocytomas, although rare, are distinctive and important to differentiate from other small, round, blue-cell tumors, oligodendrogliomas and ependymomas. Smears excel in the analysis. These tumors arise primarily in younger adults and most often present in patients between their teenage years and their forties. Given the demonstrably slow growth and large size of some of these neoplasms, it is likely that they have been present a very long time. Typically, a patient will present with effects of increased intracranial pressure, including headaches and visual disturbances from papilledema. Incidental cases occasionally surprise the neuroradiologist, for example, when a passenger in a motor vehicle accident receives a brain scan.
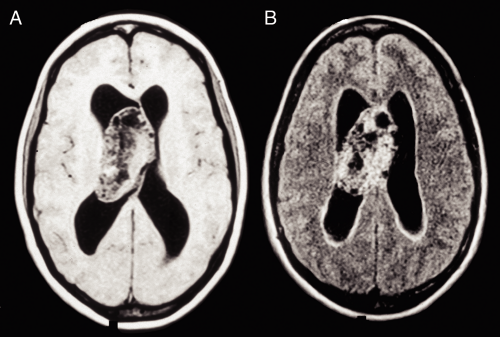
On neuroimaging, these tumors grow on or near the midline, typically protrude into the ventricular system near the foramen of Monro, and arise from either the septum pellucidum or the immediately surrounding ependymal
surfaces. Tumors outside this region technically are not “central” neurocytomas but just neurocytomas. Many of these slowly-growing tumors reach astonishingly bulky sizes before presentation (Figure 8-7). They also enhance and frequently have cystic components and calcifications. When identified in patients with neurological symptoms, they often have produced significant hydrocephalus in imaging.
surfaces. Tumors outside this region technically are not “central” neurocytomas but just neurocytomas. Many of these slowly-growing tumors reach astonishingly bulky sizes before presentation (Figure 8-7). They also enhance and frequently have cystic components and calcifications. When identified in patients with neurological symptoms, they often have produced significant hydrocephalus in imaging.
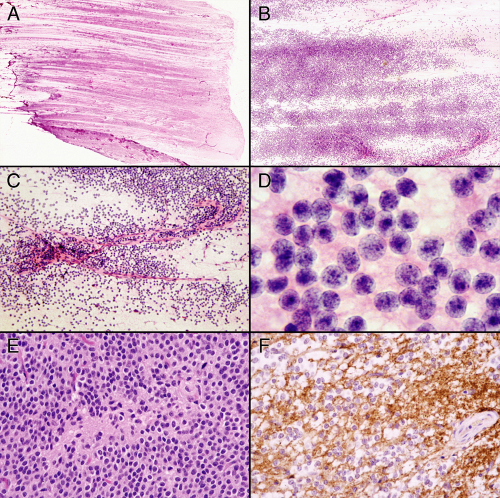
Central neurocytomas have two defining characteristics: their monotonous population of granular neuronlike cells and their loose neuropil matrix. Glial elements, although often present, do not overwhelm the neuronal basis of this tumor. A smear excels at analyzing these tumors because it best displays the cellular monotony, nuclear cytology, lack of cellular cohesion, and the light neuropil matrix. By just putting the freshly smeared slide on a white piece of paper, the tumor shows nearly liquid growth (Figure 8-8). The blue cells spread evenly across the glass. Higher magnification reveals little else about the tumor cells. Cytoplasm is scant or absent, the cells show minimal cohesiveness, and the nuclei are remarkable for their monotony. Well-formed capillaries and small vessels lack connections to the surrounding tumor cells. Only the highest microscopic powers reveal the tumor’s neuropil matrix and its nearly circular nuclei with their fine salt-and-pepper chromatin, and seeming absence of cytoplasm. Permanent paraffin-embedded sections take a step back in quality. The artifacts of processing inflict distortion in the nuclei and often give perinuclear halos reminiscent of oligodendrogliomas. On permanent sections, these tumors also produce larger regions of neuropil devoid of cells, which may easily be mistaken for ependymal pseudorosettes. As befits their origin from neural precursors, these tumors strongly express synaptophysin.
Several different tumors share aspects of central neurocytomas. Other neurocytomas (e.g., pineocytoma) look identical to this tumor and can only be “distinguished” by their location. For many years, the tumor’s perinuclear halos on permanent sections lead them to be diagnosed as intraventricular oligodendrogliomas. Although oligodendrogliomas also produce a smear of relatively round nuclei in a scant matrix, they invariably show some nuclear pleomorphism and have accompanying glial fibers and an occasional astrocyte. Unlike a neurocytoma, oligodendrogliomas




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree