Intraoperative Evoked Potential Monitoring
Alan D. Legatt
Sensory evoked potentials (EPs) are the electrical signals produced by the nervous system in response to a sensory stimulus. Auditory, visual, and somatosensory EPs are used as diagnostic tests to noninvasively assess sensory pathways within the central and peripheral nervous system, as well as to assess patients for diseases of the ear and eye. Somatosensory evoked potentials (SEPs) and auditory evoked potentials (AEPs) have also found wide utility in intraoperative monitoring (IOM) to detect neural compromise during surgery, when the patient is anesthetized and a conventional neurologic examination cannot be performed. The anesthetic sensitivity of visual EPs limits their utility for IOM.
The central motor tracts can also be monitored by stimulating them rostrally and recording from either muscles or neural structures caudal to the part of the nervous system that is at risk. By analogy to sensory EPs, the signals that are recorded are typically called motor evoked potentials (MEPs). The brain can be stimulated either electrically or magnetically in order to elicit MEPs. As discussed in Chapter 52, MEPs to magnetic stimulation are difficult to record consistently in anesthetized patients during surgery; intraoperative MEP monitoring is therefore typically performed using transcranial electrical stimulation (TES) of the brain.
The EP modalities commonly used for IOM are described in this chapter. For the sensory EPs, the description includes the anatomical generators of the components that are useful for IOM. Recording techniques, assessment of the IOM data, causes of adverse EP changes, and typical intraoperative applications are described.
FACTORS COMMON TO MULTIPLE MODALITIES
Recording Techniques
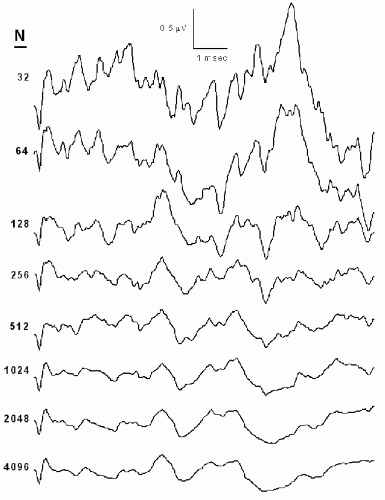
Sensory EPs are in general too small to be seen in the raw data following a single stimulus. Signal averaging is therefore employed—multiple stimuli are delivered and the data epochs or sweeps recorded following the individual stimuli are averaged together, after rejecting those that contain large artifacts. Signal averaging (Fig. 38.1) improves the signal-to-noise ratio of the averaged EP waveforms by a factor equal to the square root of the number of sweeps included in the average (1). If the EP data are noisy and/or the EP amplitudes are small, the number of sweeps per average may have to be increased. Conversely, if the signal-to-noise ratio of the raw data is favorable, the number of sweeps per average can be decreased, allowing averages to be acquired more frequently and thus facilitating more rapid notification to the surgeons should adverse EP changes occur.
EPs should only be recorded to unilateral stimulation; if simultaneous bilateral stimulation were employed, the presence of a normal EP to stimulation of one side might prevent recognition of an abnormal or absent EP to stimulation of the other side. Modern EP recording systems permit interleaving of sensory stimuli (e.g., auditory stimuli are delivered alternately to the left and to the right ears). The data sweeps are then sorted (after artifact rejection) into separate left- and right-sided averages. Thus, even though left- and right-sided averages are acquired simultaneously, simultaneous bilateral stimulation is never actually delivered.
Assessment of IOM Data
Sensory EP amplitudes vary substantially across different subjects, whereas component latencies are much more consistent. Therefore, interpretation of extraoperative EP studies performed for diagnostic purposes is predominantly based on latencies (2,3). However, EP component amplitudes on repeated recordings in the same subject are usually quite consistent if the recording techniques are not altered. Moreover, if parts of the nervous system are compromised, amplitude changes may occur earlier than, or in the absence of, latency changes. Therefore, both the amplitudes and latencies should be assessed during IOM of SEPs and AEPs.
For extraoperative sensory EP studies performed for diagnostic purposes, the patient’s signals are compared to those recorded in a control population of normal subjects. In order for this comparison to be valid, the techniques used and the state of the subjects must be identical in the patient’s study and in the control studies. During IOM, the anesthetic regimen, the body temperature, the delivered stimulus intensity, and other factors will differ from patient to patient. Therefore, during IOM each patient serves as his or her own control; EPs recorded during the time when parts of the nervous system are at risk are compared to those recorded earlier during the same operation (4). If the anesthetic regimen or other factors that will affect the EP data are changed, the baseline amplitude and latency values should be reassessed.
Causes of Adverse EP Changes
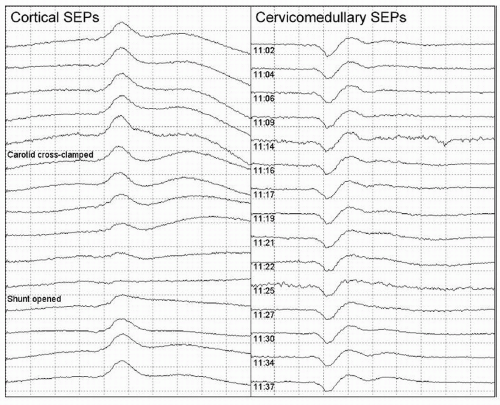
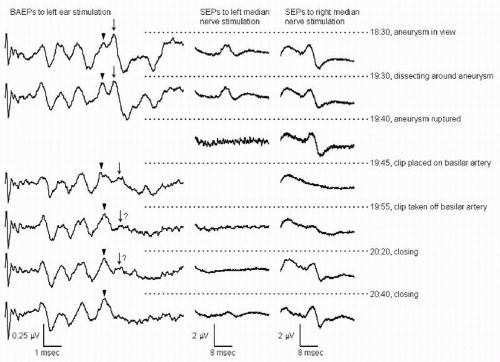
Changes in the signals recorded during IOM can be classified into three categories: “true positive” changes, which reflect compromise of the structures that the monitoring is intended to safeguard (e.g., Figs. 38.2 and 38.3); changes produced by other physiologic mechanisms such as anesthetic effects (e.g.,
Figs. 38.4 and 38.5) or hypothermia; and changes due to technical problems (e.g., Fig. 38.6) rather than to actual changes in neuronal function (5). True positive changes can reflect tissue damage due to direct mechanical or thermal injury. They can also reflect ischemia caused by compromise of the blood supply to the neural tissue (Figs. 38.2 and 38.3), by vasospasm, by generalized hypotension, or by a combination of these acting synergistically.
Figs. 38.4 and 38.5) or hypothermia; and changes due to technical problems (e.g., Fig. 38.6) rather than to actual changes in neuronal function (5). True positive changes can reflect tissue damage due to direct mechanical or thermal injury. They can also reflect ischemia caused by compromise of the blood supply to the neural tissue (Figs. 38.2 and 38.3), by vasospasm, by generalized hypotension, or by a combination of these acting synergistically.
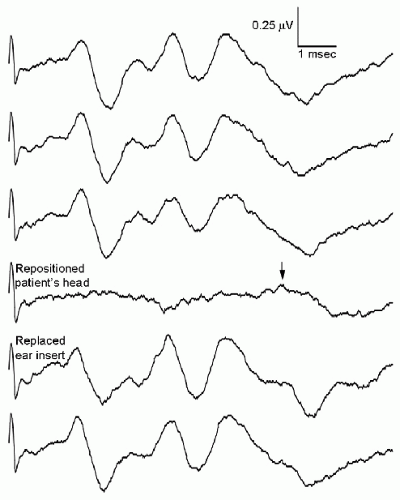
EPs can be lost due to technical problems such as equipment malfunction, dislodged electrodes or stimulators (Fig. 38.6), disconnected or broken wires, and operator error (the use of incorrect protocols or settings). A variety of artifacts can obscure the EPs (Fig. 38.7). The artifact from the cavitational ultrasonic suction aspirator (CUSA) device (Fig. 38.7C) deserves special mention, as it may manifest as a low-voltage, high-frequency artifact that obscures the EP but does not trigger automatic artifact rejection (4). Monopolar cautery use can saturate the EP recording system’s input amplifiers, preventing recording of valid EP data for some time after the cautery is stopped.
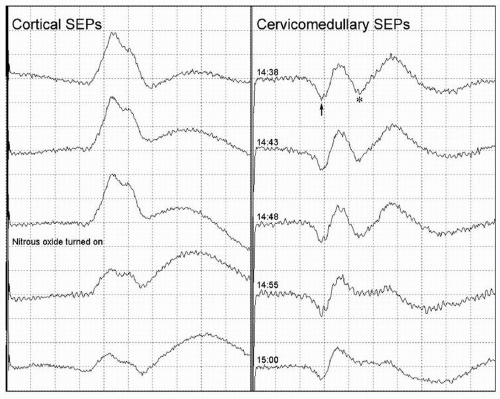
Anesthesia (Figs. 38.4 and 38.5), systemic hypothermia, and hypotension can produce EP changes that do in fact reflect changes in neural function but are different from the neural dysfunction due to surgical manipulations that the IOM was specifically intended to detect. Irrigation of the surgical field with cold fluids can produce localized tissue hypothermia, causing EP changes that resemble those that would be caused by surgical compromise of the neural tissue there (Fig. 38.8).
AUDITORY EVOKED POTENTIALS
The earliest electrical signals recorded following a transient auditory stimulus are generated within the cochlea and are recorded as part of the electrocochleogram. The subsequent neurally generated signals have been divided into latency classes—short-latency AEPs, with latencies of under 10 msec; long-latency AEPs, with latencies over 50 msec; and middle-latency AEPs, with intermediate latencies. The long-latency AEPs are generated within cerebral cortex, including cortical association areas, and are profoundly affected by the degree to which the subject is attending to the auditory stimulus and extracting information from it. The long-latency AEPs are suppressed by surgical anesthesia, and thus are not useful for IOM.
Middle-latency AEPs are also generated within cerebral cortex, including primary auditory cortex and surrounding areas (6). They are also markedly affected by surgical anesthesia, which limits their utility for IOM to detect surgery-related focal neurologic dysfunction. However, this anesthetic sensitivity has led to attempts to use middle-latency AEPs as an indicator of the depth of anesthesia (7).
Middle-latency AEPs are also generated within cerebral cortex, including primary auditory cortex and surrounding areas (6). They are also markedly affected by surgical anesthesia, which limits their utility for IOM to detect surgery-related focal neurologic dysfunction. However, this anesthetic sensitivity has led to attempts to use middle-latency AEPs as an indicator of the depth of anesthesia (7).
The short-latency AEPs are the most useful AEPs for neurologic and otologic diagnosis because they are relatively easy to record and their waveforms and latencies are highly consistent across normal subjects (8). They are also useful for IOM because surgical anesthesia produces only minor changes in them (5,9,10); some of the intraoperative changes that are observed in them may be due more to changes in body temperature than to anesthetic effects (11, 12 and 13). Although the earliest components are generated, at least in part, in the eighth nerve, most of the components are generated in the parts of the brainstem auditory pathways, and the short-latency AEPs are most commonly referred to as brainstem auditory evoked potentials (BAEPs).
Components, Generators, and Recording Electrode Locations
BAEP components are typically recorded between the vertex (position “Cz” of the International 10-20 System) and both earlobes or both mastoids (labeled “Ai” or “Mi” for the ipsilateral ear and mastoid, respectively, and “Ac” or “Mc” for those contralateral to the stimulated ear); recording the two channels provides a measure of redundancy in case one of the ear/mastoid electrodes becomes unusable during surgery and may also help in the identification of wave V (see next paragraph). Positive BAEP peaks are most often displayed as upward deflections (the opposite polarity convention from SEPs and from electroencephalography and electromyography, in which negativity is displayed as an upward deflection), and the positive peaks in the Cz-Ai recording channel are typically labeled with Roman numerals according to the convention of Jewett and Williston (14) (Fig. 38.9). Most of the BAEP components are recorded from the scalp as far-field potentials, which means that small displacements of the recording electrodes do not significantly alter the BAEP waveform.
Thus, if a CPz electrode is being placed to record lower limb SEPs, it can be used in place of the Cz electrode to record BAEPs, with minimal effect on the waveforms obtained. Wave I, however, is recorded as a near-field potential on the surface of the head in the vicinity of the stimulated ear.
Thus, if a CPz electrode is being placed to record lower limb SEPs, it can be used in place of the Cz electrode to record BAEPs, with minimal effect on the waveforms obtained. Wave I, however, is recorded as a near-field potential on the surface of the head in the vicinity of the stimulated ear.
Waves I, III, and V are the most consistent BAEP peaks, and those upon which interpretation of both extraoperative BAEP studies and intraoperative BAEP monitoring is based. Wave I is recorded as a negativity by the ipsilateral ear/mastoid electrode and is largely absent in the Cz-Ac recording (see Fig. 38.9). Wave III is typically seen in both channels, though it is smaller in Cz-Ac than in the Cz-Ai recording. Waves IV and V are seen in both channels at similar amplitudes and typically overlap, forming a IV-V complex with variable morphology (15) (Fig. 38.10). In the Cz-Ac channel, the latency of wave IV is typically a little shorter than that in the Cz-Ai channel, and that of wave V is typically a little longer (see Fig. 38.9). The increased separation of waves IV and V in the Cz-Ac channel may make wave V easier to identify in that channel.
Wave I originates in the first volley of action potentials in the auditory nerve as it begins in the most distal portion of the nerve (16). It represents the same electrical phenomenon as the N1 component of the eighth nerve compound action potential in the electrocohleogram (17), and at the skin surface, it appears as a negativity in a circumscribed area around the stimulated ear (18,19). The negativity picked up by the Ai electrode gives rise to the upgoing (positive) peak in the Cz-Ai recording. Since wave I is recorded as a near-field potential around the stimulated ear, repositioning of the Ai/Mi recording electrode can substantially alter it, and alternate electrode positions can be used to obtain a clearer wave I. An electrode within the external auditory canal may pick up an even larger wave I. Because wave I arises from the
most distal portion of the auditory nerve, it may persist after the nerve is sectioned at a more proximal location, such as during surgery for eighth nerve tumors (20,21) (Fig. 38.11).
most distal portion of the auditory nerve, it may persist after the nerve is sectioned at a more proximal location, such as during surgery for eighth nerve tumors (20,21) (Fig. 38.11).
Subsequent BAEP components are the composites of contributions of multiple generators. The complexity of the generators of human BAEPs derives in part from the anatomy of the brainstem auditory pathways, with ascending fibers both synapsing in and bypassing various relay nuclei (22, 23 and 24). It also reflects the presence of at least two bursts of activity in the auditory nerve (corresponding to the N1 and N2 components of the eighth nerve compound action potentials in the electrocochleogram), which can drive the more rostral pathways. Because of both of these factors, several different structures within the infratentorial auditory pathways may be active simultaneously, and thus contribute to the same BAEP component (16).
Wave II originates, in part, in the first auditory nerve volley (the N1 component of the eighth nerve compound action potential, which gave rise to wave I when it began in the distal eighth nerve) that has propagated to the proximal end of the auditory nerve and to the cochlear nucleus. However, this occurs simultaneously with the onset of the second auditory nerve volley (the N2 component of the eighth nerve compound action potential) in the distal nerve (17). The latter contributes to the scalp-recorded BAEP in the same manner as the N1 component did when it was at the same location. This can cause persistence of a wave II in cases where the proximal eighth nerve has been destroyed (8).
Wave III predominantly originates in the caudal pontine tegmentum, including the region of the superior olivary complex (8). Since ascending projections from the cochlear nucleus are bilateral, wave III may receive contributions from brainstem auditory structures both ipsilateral and contralateral to the stimulated ear. In patients with asymmetrical lesions of the brainstem, wave III abnormalities are usually most pronounced following stimulation of the ear ipsilateral to the lesion (25, 26 and 27), though occasionally they are more pronounced following contralateral stimulation (28).
The anatomical generators of waves IV and V are most likely in close anatomical proximity, since they are usually either both
affected or both unaffected by brainstem lesions (28, 29 and 30). They may, however, be differentially affected (16,28,31), including by intraoperative brainstem damage (Fig. 38.12). Wave IV appears to reflect activity predominantly in ascending auditory fibers within the dorsal and rostral pons, just caudal to the inferior colliculus, whereas wave V predominantly reflects activity at the level of the inferior colliculus, perhaps including activity in the rostral portion of the lateral lemniscus as it terminates in the inferior colliculus (8). As is the case with wave III, wave V abnormalities due to unilateral brainstem lesions are usually most pronounced following stimulation of the ear ipsilateral to the lesion (25, 26 and 27,32,33), though there are exceptions (34,35).
affected or both unaffected by brainstem lesions (28, 29 and 30). They may, however, be differentially affected (16,28,31), including by intraoperative brainstem damage (Fig. 38.12). Wave IV appears to reflect activity predominantly in ascending auditory fibers within the dorsal and rostral pons, just caudal to the inferior colliculus, whereas wave V predominantly reflects activity at the level of the inferior colliculus, perhaps including activity in the rostral portion of the lateral lemniscus as it terminates in the inferior colliculus (8). As is the case with wave III, wave V abnormalities due to unilateral brainstem lesions are usually most pronounced following stimulation of the ear ipsilateral to the lesion (25, 26 and 27,32,33), though there are exceptions (34,35).
Waves VI and VII are absent in some normal subjects. While they may in part reflect activity in more rostral structures such as the medial geniculate nucleus, they also receive contributions from activity in the inferior colliculus (8); the latter generator may cause persistence of these waves in patients with auditory pathway damage rostral to the inferior colliculus. Therefore, BAEPs cannot be used to assess or monitor the auditory pathways rostral to the mesencephalon.
Recording Techniques
Acoustic clicks, produced by delivering trains of 100-µsec-duration electrical square pulses to the acoustic transducer, are most often used to elicit BAEPs for IOM; brief tone pips can also be used. Since the responses to rarefaction and compression clicks may differ (36,37), extraoperative diagnostic BAEP studies typically employ a single click polarity. Stimulation with alternating click polarities and averaging the responses to rarefaction and compression clicks together is useful to cancel a large stimulus artifact and/or the cochlear microphonic and is often employed during intraoperative BAEP monitoring.
Since headphones would be impractical for IOM, the acoustic stimuli are typically delivered using ear inserts, incorporating foam cylinders that can be compressed and then gradually expand to achieve a tight fit with the ear canal. The foam can be covered with a thin layer of metal foil, to serve as a near-field electrode for recording of wave I from the external auditory canal. The ear insert can be held in place with bone wax or cotton padding, and secured with nonporous tape or an adhesive waterproof dressing pad (4,38). This is to prevent fluids from entering the ear canal, where they might interfere with transmission of sound to the inner ear. The ear insert is connected to the acoustic transducer using a segment of plastic tubing. Care should be taken to ensure that this tubing remains in place during positioning of the patient and is not kinked. The time required for the acoustic signal to propagate through the tubing typically prolongs the latencies of all BAEP components by approximately 0.9 to 1.0 msec as compared to those recorded using headphones. This causes no problems in the evaluation of the BAEPs, since each patient serves as his or her own control and the acoustic propagation delay is constant. Moreover, the delay helps to prevent obscuration of wave I by the electrical stimulus artifact, because (i) it prolongs the latency of wave I,
helping to separate it in time from the electrical stimulus artifact (which remains simultaneous with the activation of the acoustic transducer), and (ii) it permits increasing the distance between the acoustic transducer and the recording electrodes, reducing the amplitude of the electrical stimulus artifact.
helping to separate it in time from the electrical stimulus artifact (which remains simultaneous with the activation of the acoustic transducer), and (ii) it permits increasing the distance between the acoustic transducer and the recording electrodes, reducing the amplitude of the electrical stimulus artifact.
The stimulus intensity delivered during intraoperative BAEP monitoring cannot be precisely controlled, due to variability in the positioning of the ear insert, but this does not cause a problem since each patient serves as his or her own control. The intensity setting chosen should be loud enough to produce a clear BAEP but not loud enough to cause ear damage. A stimulus rate of approximately 10 per second is typical, but a rate of exactly 10 Hz or another submultiple of the power line frequency should be avoided; if such a submultiple were chosen, signal averaging would not eliminate line-frequency artifact that is present in the data. Contralateral white noise masking, such as is typically used during extraoperative diagnostic BAEP studies to prevent acoustic crosstalk, cannot be used during IOM if left and right ear stimuli are interleaved. This is not a problem because acoustic crosstalk is much smaller with ear-insert transducers than with headphones (39). Also, the major reason for white noise masking is to prevent acoustic crosstalk from producing a BAEP when a deaf ear is stimulated during a diagnostic BAEP study; absence of white noise masking will not prevent recognition of new auditory pathway compromise when a functioning ear is stimulated.
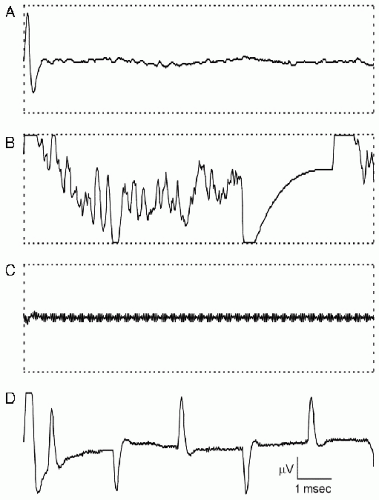 Figure 38.7 Single (unaveraged) data epochs recorded during intraoperative BAEP monitoring. A: An electrical stimulus artifact is present at the beginning of the epoch. The BAEP, which is less than 1 µV in amplitude, is not visible in this single epoch of raw data. B: Bipolar cautery produces a large artifact that triggers automatic artifact rejection. C: The cavitational ultrasonic surgical aspirator (CUSA) device produces a very high-frequency but low-voltage artifact that completely obliterates the neurophysiologic data but does not trigger automatic artifact rejection. D: The light source of the operating microscope produces a repetitive, sharply contoured artifact that recurs at a harmonic of the line frequency but is composed of higher frequencies that would not be removed by a line-frequency notch filter. In A, B, and C, the horizontal dotted lines show the input window of the analog-to-digital converter and the threshold for automatic artifact rejection. In D, the amplifier gain was reduced to show the light source artifacts in their entirety. Voltage calibration bar: 10 µV in A, B, and C, and 40 µV in D. (Reprinted from Legatt AD. Mechanisms of intraoperative brainstem auditory evoked potential changes. J Clin Neurophysiol. 2002;19:396-408.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|