CHAPTER 120 Intraoperative Magnetic Resonance Imaging
The field of neurosurgery has always been one in which the accuracy of its procedures is closely linked to the advances in neuroimaging that accompany it. The introduction of the surgical microscope was paramount in the evolution of neurosurgical technique because it increased the precision of interventions beyond the limitations of the naked eye.1 Later, frame-based and frameless stereotactic systems using preoperative computed tomography (CT) and magnetic resonance imaging (MRI) provided three-dimensional maps for neuronavigation, aiding greatly in the exploration of the brain.2 MRI in particular was appealing in the imaging of cortical structures because it had unparalleled soft tissue resolution, multiple contrast mechanisms, and the ability to acquire tomographic images in multiple planes.3,4 Irrespective of these benefits, however, the use of preoperatively obtained images to guide stereotactic navigation results in discrepancies between the virtually mapped space and actual anatomy in real time.
These inconsistencies in neuronavigation are largely a result of “brain shift,” or the deformation of cortical and subcortical structures secondary to loss of cerebrospinal fluid (CSF), tissue resection, patient position, edema, and other factors that alter the intraoperative brain environment from its previous state during preoperative scanning.4–8 Although such changes resulting from dural opening are minimal, they slowly progress with time, leading to increased stereotactic errors as the operation continues.5 To keep up with these dynamic shifts in cortical anatomy throughout surgery, a high-resolution, intraoperative imaging solution that could appreciate these changes and redirect navigation was needed. Intraoperative MRI (iMRI) was developed for this purpose.
iMRI has been used for only a little more than a decade. As with all new technologies, the beginning years of iMRI consisted of working out the setup and protocols to perform MRI-guided procedures within the operating theater.9,10 In their infancy, iMRI systems were able to improve the efficacy of surgical resection and monitoring for intraoperative complications such as ischemia, acute hemorrhage, and diffuse brain edema.1 As they became increasingly present in the neurosurgical operating room (OR), it was necessary to ask if these results translated into better patient outcomes and, if so, for which procedures was this benefit significant.11 The answer to this question is ever changing as the technology of iMRI continues to evolve. What may have been a small or undetectable benefit with the first generation of iMRI systems, owing to limitations in magnet field strength, system design, or the reporting of results, may not be applicable in the future. With the transition from low-field iMRI systems with poorer imaging quality and acquisition techniques to systems with high-field magnets, the degree of precision and the indications for which iMRI appears to be useful may continue to increase. As iMRI systems continue to improve in their ease of use and resolution, further studies will be needed to determine the extent to which iMRI is necessary in the neurosurgical OR.
Development of Intraoperative Magnetic Resonance Imaging
Since the development of the first iMRI system in 1995,9 various arrangements have emergedwith differing permutations in magnet strength, patient access, and OR suite setupbecause no one design has proved superior to all others. Currently, iMRI systems consist of magnets that range in field strength from 0.12 to 3.0 tesla (T) and are often subdivided accordingly into low-field (0.12 to 0.5 T) and high-field (1.5 and 3.0 T) systems. Although the high-field configurations enjoy superior image quality and advanced imaging capabilities, such as functional MRI (fMRI) and diffusion tensor imaging (DTI), in exchange, they sometimes sacrifice surgical access to the patient or time by requiring patient transport within the OR or to an adjacent but separate MRI suite.12 Conversely, low-field systems emphasize patient access and real-time imaging in the truest sense because many of these configurations do not require patient movement; however, their imaging resolution and capabilities are somewhat limited.9
Custom Intraoperative Magnet Designs
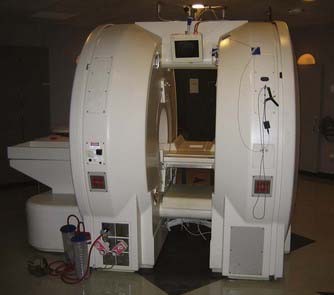
The first iMRI system was developed through a collaborative effort between physicians at Brigham and Women’s Hospital in Boston and engineers at General Electric Medical Systems. The design for the new magnet was paramount in the evolution of iMRI because it strayed from the traditional closed-coil configurations that prohibited patient access during scanning.9 Two superconducting magnets with bores 60 cm in diameter were vertically oriented in parallel, creating a 56-cm-wide gap between the magnets that provided nearly full access to the patient on either side of the OR table. The Signa SP design (General Electric Medical Systems, Milwaukee, WI), more commonly known as the “double doughnut,” positioned the patient’s head within the 0.5-T imaging isocenter located both between the two magnets and between the two surgical access sites, allowing simultaneous imaging and surgery without the need to move the patient (Fig. 120-1). The scanner also had a three-dimensional navigational system (Flashpoint, Integrated Technologies, Boulder, CO) that provided tracking capabilities within the imaging area. Because surgery took place within the magnetic field, MRI-compatible surgical and anesthesia equipment was required. Also of note was a specially constructed MRI-compatible microscope that provided the magnification necessary for neurosurgery but did not have many of the features offered on other neurosurgical microscopes, such as autofocus or autozoom.3 As the first of its kind, this iMRI system offered the unprecedented ability of producing near real-time intraoperative magnetic resonance images without the need to move the patient or magnet, while still providing adequate surgical access. The disadvantages included lower image quality relative to systems with high-field magnets, the need for more costly magnetic resonance–compatible equipment, and the somewhat limited surgical maneuverability given the confines of the magnets.1
After the debut of the Signa SP into the neurosurgical world, other iMRI systems emerged that used novel magnet designs to optimize patient access with intraoperative imaging. The PoleStar magnet (Odin Medical Technologies, Yokneam, Israel) was one such design, which consisted of two vertical parallel disks containing ceramic magnets placed 25 cm apart and connected by a U-shaped arm that wrapped around the patient’s head from below (Fig. 120-2). The portability of the magnet limited its field intensity (0.12 to 0.15 T) but granted it great compatibility within the traditional OR. Given its low field strength, traditional surgical tools and equipment could be used and, if needed, the magnet could be placed inside its iron storage cabinet to virtually eliminate the magnetic field within the OR. Its small size allowed it to be wheeled to and placed beneath the patient’s head when needed for scanning, without any additional floor reinforcement. An optional radiofrequency (RF) shield that extended around the patient and magnet like a tent during imaging was available for ORs that were not RF shielded. Moreover, the surgical navigation system and the scanner could be operated by the neurosurgeon or OR staff, obviating the need for a neuroradiologist or a specific MRI technician.3,13
The physicians and scientists from Toronto Western Hospital developed a vertical gap system that took the first step toward a mobile patient model in the evolution of iMRI. This system consisted of a traditional 0.2-T horizontal gap permanent magnet system that was rotated 90 degrees to create a vertical gap. While the patient was positioned within the magnet’s isocenter, simple procedures such as catheter and shunt insertions could be performed by the single surgeon who stood within the vertical gap. By sliding the patient, head first, 1 to 1.5 meters away from the scanning position, a surgical position with 270 degrees of access could be achieved. Throughout the movement of the patient, overhead cameras maintained patient registration, allowing shifts between surgery and scanning without the need for reregistering anatomic coordinates to magnetic resonance images.3 Despite its decommissioning in 2003, the 5-year trial with this vertical gap system demonstrated that moving the patient in and out of the scanner was not the serious hindrance it was once believed to be. From this experience, others began to explore the possibility of combining a high-field closed-bore MRI system, which would provide superior image quality, with this efficient means of switching between scanning and performing surgery.
Adaptations of Traditional Magnetic Resonance Imaging System Magnets
In addition to the custom MRI system magnets specially designed for intraoperative use, some iMRI systems incorporated preexisting low-field or high-field scanners into a modified OR environment. Horizontal gap systems, such as the Hitachi AIRIS I and II (Hitachi Medical Systems, Twinsburg, OH, and Kashiba, Chiba, Japan) and the Magnetom Open Viva (Siemens Medical Solutions, Erlangen, Germany), consisted of traditional low-field scanners with a single, integrated MRI-compatible OR table (Fig. 120-3). The table was modified so that it could rotate the patient’s head away from the magnetic isocenter and beyond the 5-Gauss (5G) line, where standard surgical instruments could be used. These iMRI systems were an extension of work initially done at the University of California, Los Angeles (UCLA), which first suggested that in addition to brain biopsies10 and transsphenoidal surgeries14 performed beyond the magnet’s 5G line, full craniotomies and surgeries could be safely conducted there as well.15 Monitoring and surgical devices that were needed within the isocenter zone (e.g., anesthesia equipment, electrocardiogram leads, and tools for interventional procedures) needed to be magnetic resonance compatible, whereas routine surgical instruments could be used in the outer zone. The patient transfer between imaging and surgery took only a few minutes, and sometimes the magnet was shared as a diagnostic MRI unit as well, thereby reducing overall costs. Despite the low-field nature of these systems (0.3 T), many investigators who have used them have reported their definite utility in the resection of gliomas and pituitary tumors and have even argued that they may be comparable to their high-field counterparts.16,17
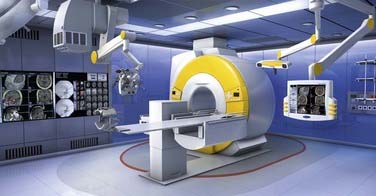
High-field systems such as the BrainSUITE (BrainLAB, Feldkirchen, Germany) use the same rotating MRI system OR table but with conventional 60- or 70-cm closed-bore magnets, Magnetom Sonata Maestro Class and Magnetom Espree (Siemens Medical Systems), respectively (Fig. 120-4). These 1.5-T systems afford the same degree of patient access and ease of transition between the magnetic and surgical fields while providing superior imaging quality and advanced acquisition techniques, such as fMRI, diffusion-weighted imaging (DWI), DTI, magnetic resonance angiography (MRA), magnetic resonance venography (MRV), and magnetic resonance spectroscopy (MRS).12
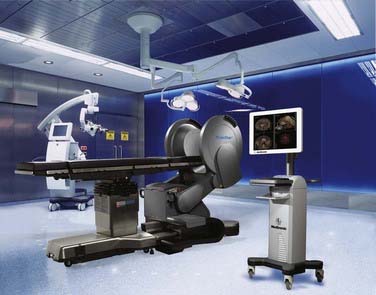
Other innovations in modifying the OR suite to accommodate intraoperative imaging included the rail-mounted system (Fig. 120-5), which was first implemented in Calgary, Canada (IMRIS, Calgary, AB, Canada). This system emphasized the preservation of the traditional neurosurgical operating theater, arguing that many compromises were made to accommodate preexisting iMRI systems, including the need for custom magnetic resonance–compatible surgical equipment, restricted patient access, and the need to transport the patient within the OR or to an adjacent imaging room. Storing the magnet in a separate room not only permits use of the OR as a traditional neurosurgical OR but also allows the MRI unit to be used in other surgical suites or for diagnostic purposes. The 1.5-T closed-bore magnet can be moved from its resting site outside the OR into position and ready to image in less than 90 seconds. All magnetic resonance–incompatible surgical tools and equipment are moved outside the 5G line and powered down during imaging. This transition has been completed in as short a time as 10 to 12 minutes.3
Aside from the original concept of conducting intraoperative imaging in the same time and space as conventional MRI, there have been many twin operating theater models in which the MRI system is located in an adjacent but otherwise separate room. This setup was first described by Tronnier and colleagues10 and was actually developed soon after the original double-doughnut model. The clear advantages of having a specialized MRI suite apart from the operating theater include being able to use MRI-incompatible surgical tools and equipment, having full patient access in a traditional surgical environment, and being able to use the MRI equipment in multiple ORs and departments, thereby greatly reducing overall costs.3,16 In one such 3-T system, the patient is manually slid onto a modified MRI docking table, using a custom tabletop transfer system, and is subsequently moved down a sterile corridor into the MRI suite. The table docks with the MRI scanner, and imaging can then proceed. The portable anesthesia system that maintains sedation while the patient is away from the OR and the physiologic monitoring equipment are both MRI compatible.3
Indications for Intraoperative Magnetic Resonance Imaging
iMRI has been used in many different neurosurgical procedures since its advent over a decade ago. Because the technology is relatively new, investigators are still trying to determine the types of interventions in which the benefit gained from it will justify the increased cost and operating time. Although there have been many surgical improvements, given the aid of iMRI, it is uncertain whether these will affect patient outcomes in the long run. Some commonly accepted indications for iMRI guidance include glioma resection (especially for larger tumors and low-grade gliomas), transsphenoidal pituitary adenoma resection, resection of seizure foci in refractory epilepsy, brain biopsies, and monitoring of intraoperative complications such as hyperacute hemorrhage.4,6,11,18
Glioma Resection
Much of the work with iMRI has focused on its ability to facilitate glioma resection. As was previously discussed, traditional stereotactic neuronavigation relies on fixed preoperative MRI scans of the lesion and its surrounding anatomy. The reliability of these images slowly diminishes throughout the surgery as CSF loss, surgical maneuvers, patient positioning, edema, and tumor resection all progressively alter the environment of the brain, producing increasing image inaccuracy with time (Fig. 120-6).4–811 Neurosurgeons have been cognizant of this phenomenon of “brain shift” during volumetric stereotaxis since it was first reported by Kelly and associates in 1986.19 Although shifts of the surface cortex could be compensated for through the visualization of superficial structures and were appreciable by previously available intraoperative navigation, subcortical shift was poorly understood.8
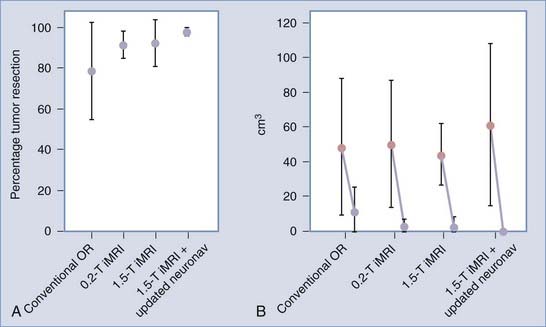
More recently, however, with the advent of iMRI and the resultant ability to better assess shifts of cortical and subcortical matter in patients within the OR, investigators have begun to quantify the magnitude and direction of brain deformation under surgical conditions. Shifts of up to 5 cm have been reported on the cortical surface and of up to 3 cm within the deep brain tissue during glioma resection, with great interpatient variability.5–8 The inaccuracy of stereotaxis is made apparent during glioma removal when the surgeon appreciates the discordance between the neuronavigation information and the appearance of the actual resection cavity. The surgeon’s inability to ascertain precisely the extent to which subcortical structures are shifted results in the overresection or, more often, underresection of lesions. With the use of iMRI, however, the neuronavigation system can be updated to agree with the intraoperative anatomy with an accuracy to within a couple of millimeters, even when using low-field systems.8,11 Reregistering the patient’s data with the navigation system using high-field iMRI has been shown to improve the consistency of intended gross-total resections (GTRs) significantly, especially with gliomas larger than 40 cm3 (Fig. 120-7).11 Although other surgical adjuncts, such as intraoperative ultrasonography, intraoperative CT, and mathematical models, have all been used to assess and even predict brain deformation, these methods still lack the resolution and compensatory ability to illustrate the changing brain structures adequately during neurosurgery.4,7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree