Recent microsurgical progress, including advancements in instrumentation, have fostered a more aggressive, successful surgical approach to intramedullary spinal cord tumors. Throughout nearly two decades, the senior neurosurgeon–author, Fred J. Epstein, M.D., has used a radical surgical approach to intramedullary gliomas on more than 200 children and 150 adults. Based on his experience and that of others, it is now an established, exciting fact that with most pediatric spinal intramedullary tumors, gross total resection can be safely accomplished. It is imperative, however, to note that early diagnosis and treatment improve postoperative outcome. The pathological type of intramedullary tumor present is reflected in the individual patient’s clinical course. Benign neoplasms typically manifest symptoms over a period of several months to years, whereas children with malignant neoplasms develop symptoms over a brief period of a few weeks to months. When a patient has a benign ependymoma that has hemorrhaged, an exception to this clinical pattern occurs as signs and symptoms develop over a brief period of hours or even a few days, similar to the time frame of a malignant tumor. Common indications for surgery include (1) motor regression, such as refusing to stand and reverting from walking to crawling; (2) local pain along the spinal axis escalating in severity at the spinal level directly over the tumor; (3) gait abnormalities reflecting lower extremity weakness; (4) dysesthesias; (5) progressive kyphoscoliosis; and (6) torticollis with corroborative radiographic images. Sensitivity and specificity make magnetic resonance imaging (MRI) the current diagnostic procedure of choice except in patients with severe scoliosis or adjacent metallic implants, which produce disturbing artifacts. In its disclosure capacities for intramedullary, rostral, or caudal tumor-associated cysts, the T1-weighted MRI is most informative; the T2-weighted MRI is reserved for assessing associated edema and for providing complementary information. In that the administration of gadolinium (Gd-DTPA) enhances the solid component of some intramedullary spinal cord tumors, delineating gadolinium-enhancing regions of the tumor from adjacent cord edema, preoperative evaluation of all patients with intramedullary spinal cord tumors must include Gd-DTPA scan. Specifically, most tumors of pediatric patients are low-grade astrocytomas and gangliogliomas. When treated with aggressive resection, approximately 85% of patients may experience long-term, progression-free survival even without additional treatment. The following serves to detail the surgical procedure used for resection of spinal intramedullary tumors. The patient is placed in the prone position. The body lies on longitudinal gel rolls on both sides, thereby allowing free chest movements and preventing abdominal compression, which would result in increased venous pressure. For cervical and upper thoracic operations, the hands are secured on the sides; for lower thoracic or lumbar procedures, they may be elevated and positioned on armboards. All pressure points must be adequately padded and cushioned. The legs are slightly elevated and wrapped with elastic bandages. All patients should have a perioperative indwelling urinary catheter. For cervical operations, the gel rolls are advanced to the edge of the bed and elevated slightly at their upper ends to create space for the chin, thus reducing the amount of reverse trendelenburg required to reach a horizontal cervical spine when the head is flexed. The skull is immobilized to the operating table using a pin-fixation device (Fig. 16–1). The surgeon(s) and assistants stand on both sides of the patient. The microscope and the CO2 laser are on one side of the table. One corner of the room is devoted to the evoked potentials team and equipment. The ultrasonic aspirator is brought in from either side. The ultrasound screen should face the operating surgeon. FIGURE 16–1. The patient is positioned prone on chest rolls, the head is immobilized in the skeletal fixation device and the neck flexed. Before scrubbing, the exact location of the spine level(s) of interest is calculated by counting either from a landmark below (i.e., the anterior superior iliac spine) or from one above (i.e., the C-7 spinous process). In the thoracic region, verification by a localizing radiograph is recommended. Following skin cleaning, the midline and a few perpendicular lines are drawn with methylene blue or an indelible marker. The epidermis and dermis are opened with a number 10 blade. The rest of the skin is opened with the monopolar cutting instrument to the level of the fascia. The skin must be opened one level above and below the planned span of the laminotomy or laminectomies. It is important to identify the fascia and to divide it in the precise midline for easy identification when one later closes the wound (Fig. 16–2). Two self-retaining cerebellar retractors are inserted, and hemostasis is obtained with the bipolar coagulation device. The spinous processes and the laminae are exposed in the standard subperiosteal fashion until the medial facets are identified. The self-retaining retractors are repositioned to a deeper level, and adequate hemostasis is obtained. FIGURE 16–2. Exposure of the laminae and spinous processes. The muscles are retracted to the sides with self-retaining retractors. Note the craniotome underneath the lamina, medial to the facet joint. The interspinous ligaments and the ligamentum flavum are resected in the midline above and below the planned span of the laminotomy flap. The epidural fat is exposed at these levels. The laminae are marked symmetrically with methylene blue at points just medial to the corresponding facet joints. The Midas Rex instrument (or any other high-speed device) is applied at the epidural plane to cut the laminae at a slightly diagonal level. The laminotomy flap is fully released and preserved. Bleeding epidural veins are carefully coagulated, and thin strips of Gelfoam are applied to the lateral gutters to assist in the tamponade of epidural oozing. Next, ultrasound is used to verify that the bony exposure is adequate to locate the intramedullary tumor (Fig. 16–3). If it is necessary to improve the exposure, the laminotomy is expanded in the appropriate rostral or caudal directions. It is unnecessary to extend the laminotomy or the dural opening over rostral and caudal tumor-associated cysts; the latter are typically decompressed after the solid portion of the tumor has been debulked. Both rostral and caudal epidural electrodes are inserted (Fig. 16–3), secured, and then connected to the monitoring unit. FIGURE 16–3. The ultrasound probe is applied to the saline-filled wound for locating the rostral and caudal ends of the tumor. The dura is best opened with a number 11 blade after it has been elevated with a dural hook (Fig. 16–4). Further opening is executed with the blade on top of a grooved dental tool. A small dissector is used to peel the dura from the adjacent arachnoid mater. Retraction sutures are applied to the cut dural edges and suspended by he-mostat clamps (Fig. 16–4). The operating microscope is brought into play. At this stage, ultrasound identifies rostral, caudal, or intratumoral cysts for determining that portion of the solid tumor with the greatest anterior-posterior dimension. Next, intradural monitoring electrodes are placed. As the bulk of the underlying mass lesion usually distorts the spinal cord, obscuring its normal anatomy, it is thoroughly examined. Both of the dorsal root entry zones are identified in an attempt to locate the precise midline. Often the midline raphe may be identified by the small diagonal vessel typically situated at the medial surface of the posterior columns. Next the midline dorsal surface of the cord is meticulously bipolar coagulated. In general, coagulation of posterior surface veins is risk free. The myelotomy incision is best initiated with an arachnoid knife in that region of the cord in which the thickest portion of the tumor exists (Fig. 16–5). Any bleeding vessels are coagulated carefully with the irrigating bipolar.
PREOPERATIVE EVALUATION
Early Indicators
Diagnosis
SURGERY
Patient Positioning and Room Arrangement
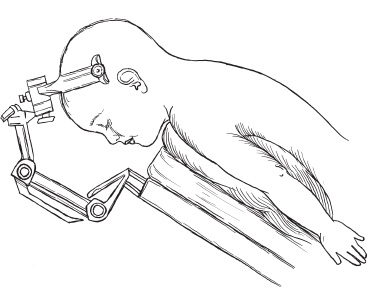
Soft-Tissue Opening
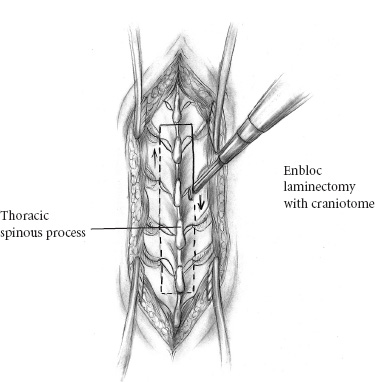
Osteoplastic Laminotomy
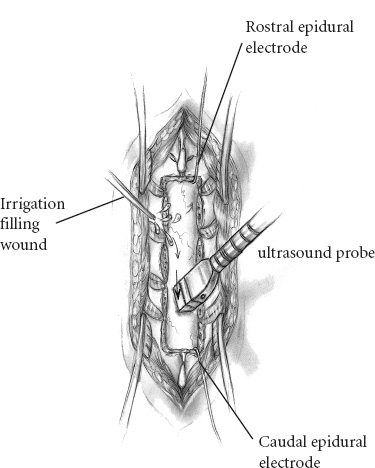
Dural Opening
Midline Myelotomy
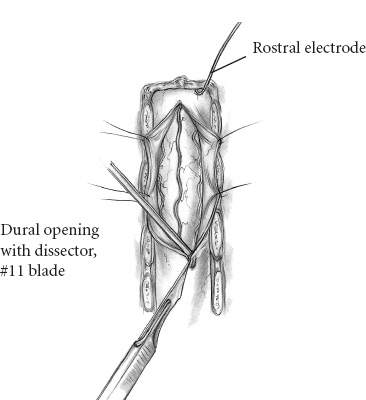
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


