You have a big chunk of tissue in the frozen section room and the surgeon needs an answer. Why bother with a smear? As anyone who has faced making an intraoperative diagnosis on a neurosurgical case knows, brain tissue is tricky to freeze well. Brain is a sea of water wrapped in tubes of fat, with a bit of protein thrown in to hold it all together. Freezing artifacts abound, especially for the unwary. Your tissue on a glass slide can look like Swiss cheese containing blue, crenated nuclei, or worse, just a roll of paper lifting up the coverslip (Figure 1.1). Although tricks and experience improve these problems, they are always a threat. At the end of the day, the tissue that was frozen and then fixed in formalin has lost valuable information: oligodendrogliomas no longer have halos, glial matrices often lose their strands, and nuclei become smudged or crenated. From that big piece of tissue, you have been forced by limitations of the cryostat into freezing only a small portion, only one section. In many difficult cases, lesions can be nearly impossible to distinguish from nearby normal brain. Your permanent slides could show gliosis next to a metastasis when the frozen section of its edge looked like a glioma. Finally, neurosurgeons are typically loath to send you a big chunk of brain. After all, unlike the tissue-greedy pathologists, they have to speak with their patients after the surgery.
Stereotactic biopsies are typically about the size of a broken piece of pencil lead. Freezing these in their entirety destroys them for their principle use: making a diagnosis on the final, highest quality histology. Freezing also precludes possible ultrastructural studies. Any additional work would require obtaining another core biopsy, each core with its own associated risk of bleeding.
With a bit of practice, smears eliminate freezing artifacts, diminish the use of precious tissue, and avoid sampling problems. Obviously, freezing artifacts disappear and you preserve more tissue for other studies when you examine only tiny pieces in the frozen section room. However, with a smear you can sample several tiny but representative areas over a larger biopsy. Some of that yellow stuff in one corner, some of the pink stuff in the middle, and some of the gray stuff on the other edge can all go on one slide for examination (Figure 1-2). This is one of the unrecognized powers of a smear, a power that utilizes the eye of the pathologist to grossly discriminate types of tissue.
Processing tissue creates its own set of artifacts. Formalin fixation coagulates proteins and can separate structures that are normally biologically apposed. The best example of this are the “halos” in classic oligodendrogliomas; these are an artifact of processing and are not present on the smear (Figure 1-3). This artifact itself has generated the decades-long battle about what constitutes an oligodendroglioma (it is in essence defined as a glial tumor with halos). After fixation, alcohols dehydrate the tissue, xylene leaches out any remaining fat, and hot wax solidifies the remnant, only later to be removed with more heat. These steps can leave smudged nuclear chromatin and reduce the fine structure of a tumor’s matrix (Figures 1-1B and 1-4).
Not only do smears eliminate or overcome many of the problems associated with freezing or processing, they also have their own benefits. Unlike all other standard histologic techniques, smears and similar preparations look at whole cells and nuclei rather than slices through them. A well-prepared smear gives invaluable nuclear detail that is lost in most standard techniques. Such detail may be critical in deciding whether a lesion is reactive or neoplastic. As mentioned above, smears use a minimal amount of tissue, which leaves larger quantities of remaining, high-quality tissue for permanent sections, immunoperoxidase studies, electron microscopy, and in the near-future, genetic studies. If a stereotactic biopsy is 10 mm long,
a smear can look at 1 mm from each end, leaving 8 mm for other studies (Figure 1.5). If entirely frozen, you are left with near garbage.
a smear can look at 1 mm from each end, leaving 8 mm for other studies (Figure 1.5). If entirely frozen, you are left with near garbage.
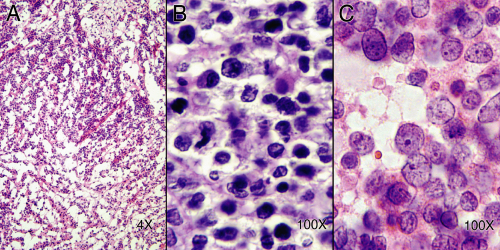 FIGURE 1-1. Freezing and processing artifacts. A. Frozen section from a spinal mass. The tissue has been “blown apart” by slowly freezing wet tissue. Although a small, round, blue cell lesion could be diagnosed, much more would be uncertain. B. Nonfrozen tissue after routine fixation and processing. The cytology is poor and detail about the tumor’s growth is lacking. C.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|





