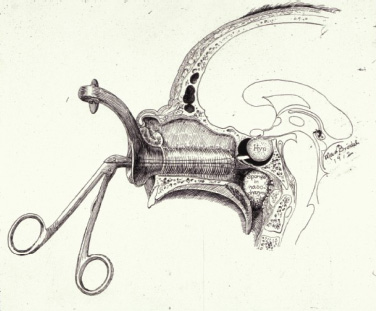
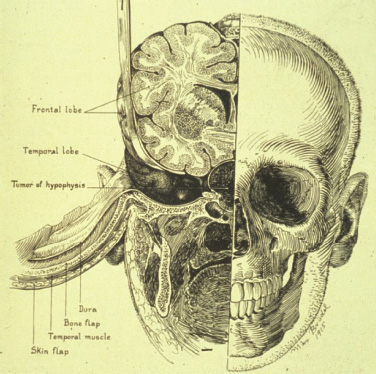
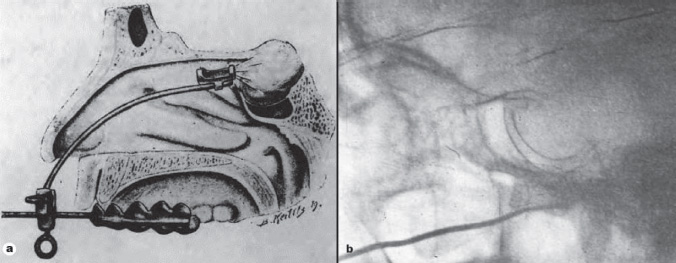
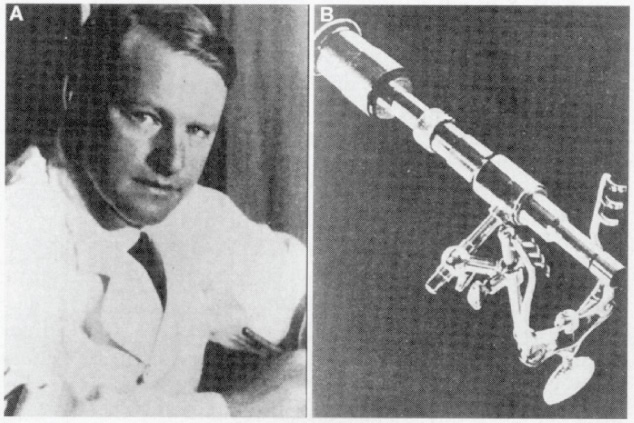
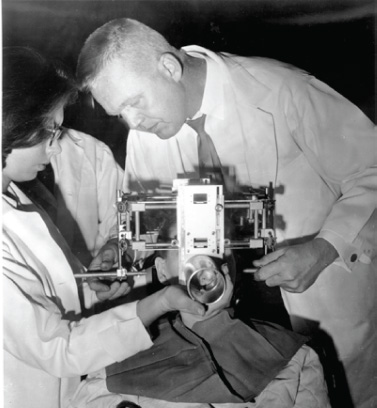
1 Introduction and Historical Perspective “As is true of all difficult operative procedures, the performance becomes progressively simplified by the combined suggestion and experience of many.” Harvey Cushing, 19121 Ever since Pierre Marie recognized acromegaly as a pituitary-related syndrome in 1886, intense vigor has characterized the search for knowledge related to the anatomy, physiology, and pathology of the pituitary gland.2 Interdisciplinary research and collaboration have allowed a dramatic transformation in the management of pathology of the sellar and parasellar region. Over the past century, collaborative efforts have resulted in medical and surgical advances that allow us to preserve normal function, restore vision, and restore to health a group of patients who were once uniformly condemned to disability and death. Initial attempts at the surgical management of pituitary lesions were made primarily in patients with large lesions who presented either with the characteristic visual loss associated with compression of the optic chiasm or with symptoms and stigmata of acromegaly. The first published cases of treatment with craniotomy by Sir Victor Horsley and others were not uniformly encouraging.3 Because surgeons were unaware of the intricacies of the hypothalamic-pituitary axis and unable to properly visualize the sella, early procedures were blunt and largely blind attempts to perform what would “generously” be called hypophysectomy. Horsley’s early attempts at subfrontal approaches were plagued by complications secondary to the extent of cerebral retraction necessary to visualize the sella. In 1893, under the encouragement of Horsley, Caton and Paul in Liverpool used a subtemporal approach in a patient with acromegaly4 (Fig. 1.1). It would be another 15 years before Schloffer attempted to use a more direct transnasal transsphenoidal route to the sella, which resulted in the patient’s death due to cerebrospinal fluid leak and meningitis.5 Not discouraged by this early result, surgeons developed a variety of more successful transsphenoidal approaches nearly simultaneously in the early 1900s. Kanavel and Kocher introduced the sublabial approach, which Cushing popularized while he was at Johns Hopkins6–8 (Fig. 1.2). Oskar Hirsch, then in Vienna, developed a transnasal transseptal approach, which became the basis for every subsequent endonasal operation for pituitary lesions.6 In 1927, near the end of his tenure at the Peter Bent Brigham Hospital in Boston, Cushing abandoned the transsphenoidal approach for craniotomy for most of these lesions (Fig. 1.3). In 1933, Hirsch relocated to Boston, continuing to use the transnasal transsphenoidal approach long after much of the growing neurosurgical community had followed Cushing’s lead.9 Hirsch recognized the limitations of both transsphenoidal and transcranial operations in completely resecting pituitary tumors. With great prescience, in 1910 Hirsch used local irradiation with an endonasal radium bomb under roentgenographic guidance as adjuvant therapy, thus beginning both image guidance and radiosurgery for sellar lesions10 (Fig. 1.4). As surgeons across the globe struggled to visualize the pituitary from both the transsphenoidal and transcranial routes, a quiet revolution was beginning in Stockholm. In 1921, Carl Nylén, an otolaryngologist at the University Clinic in Stockholm, performed the first microsurgery with a monocular dissecting microscope11 (Fig. 1.5). A year later, Gunnar Holmgren, Nylén’s chief, attached a light source to a binocular microscope. This allowed three-dimensional depth perception, and the external light source made it possible to work at even higher magnification than ambient light would.12 In 1938, two otolaryngologists, Tullio and Calicetti, working in Parma, Italy, overcame two more limitations; they mounted the microscope on a counter-weighted base to minimize vibrations and mounted a prism beam splitter in the optical pathway, which allowed two surgeons to work with the same field of view. These and other refinements were ultimately incorporated in the first generation of operative microscopes in the early 1950s, when commercial production for use in otolaryngology and ophthalmology began.12 Microneurosurgery as we know it was born on August 1, 1957, when Theodore Kurze at the University of Southern California, encouraged by William House’s feats of microscopic middle ear surgery, brought a microscope into the operating room to assist with resection of a facial nerve schwannoma.13 One year later, the first interdisciplinary microsurgical laboratory was established by R. M. P. Donaghy in Burlington, VT.14 Over the next decade, collaboration between neurosurgeons and vascular surgeons, building on the early experience of ophthalmologists and otologists, resulted in new instrumentation and techniques. These were taught to M. Gazi Yaşargil, who returned to Zurich in 1967 after a year in Donaghy’s laboratory to perform the first cerebrovascular bypass.15 With the newly found ability to illuminate and visualize deep structures, neurosurgeons and otolaryngologists began to approach the sellar and parasellar region with bravado. In the 1950s and 1960s, Smith and Ketcham’s reports of combined transfacial and transcranial approaches to the paranasal sinuses re-ignited interest in radical resection of tumors in this region.16 Parkinson reported one of the first successful microsurgical approaches to the cavernous carotid.17 Over the next 20 years, the field of microscopic neurosurgery of the skull base underwent a period of exponential growth and popularity. Aided by the anterior and middle fossa approaches described by innovators such as Dolenc, Sekhar, and Al-Mefty, and the transfacial approaches described by Smith, Ketcham, VanBuren, and others, surgeons were no longer impotent in addressing complex lesions of the sellar and parasellar regions via transcranial and transfacial approaches.18,19 Norman Dott returned to Edinburgh after spending 1923–1924 in Cushing’s clinic and surgical theater at the Brigham. In contrast to most surgeons, who followed Cushing’s lead in abandoning the transsphenoidal approach, Dott continued to practice and refine it over the next 30 years20 and demonstrated its virtues in the mid 1950s to his pupil, Gerard Guiot of France. Guiot was an extraordinary innovator, and he designed new instrumentation, including an endoscope, to facilitate the operation. However, he abandoned the endoscope because of its poor illumination and visualization compared with the operative microscope. Guiot adapted intraoperative fluoroscopy, using air and contrast to localize instruments in the sagittal plane and visualize the extent of tumor resection21 (Fig. 1.6). This allowed him and his brilliant pupil, Jules Hardy of Montreal, to safely and accurately work above the sella under indirect visualization. This marked the beginning of real-time image guidance in pituitary surgery, which continues to evolve today. While working with Guiot, Hardy began to use the operating microscope and microtechnique in pituitary surgery. In another example of simultaneous advancement, Hardy reported the use of the operating microscope for selective hypophysectomy in 1965—the same year Leonard Malis, at Mount Sinai Hospital in New York, used the operating microscope to completely resect a craniopharyngioma in a patient with a prefixed chiasm.22,23 The advancements in microsurgery introduced by Yasargil and Malis—namely, bipolar electrocautery for hemostasis and microsurgical techniques—were adapted by Hardy for pituitary surgery. Hardy’s demonstration in the late 1960s that one could do selective operations to remove pituitary microadenomas, while preserving the normal pituitary gland, was a revolutionary concept and led to renewed interest in transsphenoidal microsurgery for the treatment of pituitary tumors.24,25 The development of roentgenography in 1896 and its ability to visualize the bony structures in the region of the sella became not only a key factor in the anatomic diagnosis of pituitary disease but also the basis for the radiosurgical treatment of many pituitary disorders. In 1901, Oppenheim reported the first use of roentgenography to diagnose a pituitary tumor. By 1907, the same year that Schloeffer attempted the first transsphenoidal pituitary operation, physicians were beginning to use X-ray therapy to treat acromegaly. In 1909, Gramegna, in Venice, reported his 2-year experience treating a patient with acromegaly by directing X-rays transorally to the sella.26 That same year, Béclère reported a case in which the enlarged sella of a 16-year-old giant was irradiated with X-rays, and after the patient’s vision improved and headaches abated with irradiation alone, Béclère abandoned his plans for craniotomy.27 External beam radiation therapy was demonstrated to be a viable alternative to surgery when he reported the patient’s 5-year symptom-free follow-up in 1913. With the Curies’ discovery of polonium and radium as sources of ionizing radiation, surgeons began to experiment with crude forms of brachytherapy. In 1912, Hirsch reported his use as early as 1910 of a radium “bomb” placed transnasally, with image guidance, to treat a pituitary tumor.6,10 Cushing’s case logs for the decade from 1914 through 1923 reveal several cases in which he used brachytherapy, although he was not enthusiastic about the results.28 The end of World War II also brought several scientific and technologic advances to medicine. The cyclotron, developed by Ernest Lawrence at Berkley National Laboratory, provided not only a sustainable source of fissile material for military purposes but also a new source of high-energy protons and helium ions for use in external beam radiotherapy. Lawrence’s brother John began experimental treatments with the synchrocyclotron in patients in the early 1950s.29 These treatments of neurosurgical disease were expanded upon in the early 1960s by Kjellberg at Massachusetts General in Boston, who treated patients in the Harvard cyclotron.30 (Fig. 1.7). The end of the war also brought a new exchange of ideas, such as the fateful discussion in 1947 between Sir Hugh Cairns and Lars Leksell at the first meeting of the Scandinavian Neurosurgical Society in Oslo. Encouraged by Cairns, Leksell proceeded to develop a polar-coordinate stereotactic frame for arc-based neurosurgery.31 After early experiments, Leksell was dissatisfied with the collateral damage and imprecise therapy delivered by coupling his frame with existing X-ray and proton sources. The delivery of the first production gamma knife in 1968 to the Karolinska Institute in Stockholm ushered in the era of “radiosurgery,” a term coined by Leksell to suggest the idea of an operation in which the surgeon used a single dose of radiation as precisely as a knife to “excise” (or in this case inactivate) a lesion.31 (p 4) Over the years, stereotactic radiosurgery (gamma knife in the late 1960s, LINAC [linear accelerator] in the early 1980s, CyberKnife in the 1990s) has become an indispensable tool in both the primary and the adjuvant therapy of sellar and parasellar lesions. Although the endoscope was used with varying success as early as 1910 for intraventricular surgery, its resolution (and therefore utility) was limited in both cranial and endonasal surgery until the mid 20th century.32 Two dramatic design advances in the 1950s—the Hopkins rod-lens and Storz “cold” illumination—suddenly allowed surgeons to see with the same clarity as did an operative microscope.33 As the resolution and illumination of endoscopes improved, they were rapidly adopted by rhinologists for use in sinus surgery. A whole armamentarium of instruments and techniques was developed and refined by otorhinolaryngologists working in relative isolation from neurosurgeons until the mid 1990s, when, as with microsurgery in the 1950s, collaboration between the two disciplines launched a new era in surgical technique. In the late 1970s, the first reports of endoscopically assisted transsphenoidal surgery emerged.34,35 Over the ensuing years, improvements in lighting and digital cameras allowed the development of endoscopes with visualization besting that of a galilean instrument. Angled lenses allowed surgeons to look off axis, and combined with angled sinus instrumentation, they allowed surgeons to work in areas previously unreachable from an endonasal approach. These technologic advances made possible additional work on the suprasellar portion of tumors, as noted by Apuzzo et al in the late 1970s and by Fries and Perneczky in the late 1980s.35,36 However, most neurosurgeons continued to consider the endoscope a replacement for angled mirrors and relegated its use to augmenting the operating microscope. By the early 1990s, collaboration between neurosurgeons and otorhinolaryngologists resulted in the first reports of purely endoscopic approaches to the sella. Jho and Carrau in Pittsburgh are credited with demonstrating the safety and efficacy of a purely endoscopic approach for pituitary adenoma37,38 (Fig. 1.8). In a recapitulation of the evolution of microsurgery, they described initial attempts as single-nostril single-surgeon approaches. Nearly simultaneously, Cappabianca, de Divittis, and colleagues in Naples, Italy, reported their initial experiences with the Jho-Carrau technique, taking a rigorously scientific approach to designing improved instrumentation and addressing the limitations of the techniques.39,40 Soon thereafter, reports from Kassam, Snyderman, and Carrau also described a binasal three-to four-hand technique that restored the neurosurgeon’s ability to perform bimanual microneurosurgery.41 In the early 2000s, the combined experience of many surgeons allowed exponential improvement in the purely endoscopic technique over a relatively short time. Today, surgeons are able to attack disease from the olfactory bulb rostrally to the odontoid process caudally and as far laterally as the foramen ovale from a purely endonasal endoscopic approach. The surgeon who treats these disorders now can choose an optimal approach tailored to the patient and the disease. It may be traditional transsphenoidal microsurgery, precise craniotomy based on skull base concepts, minimally invasive eyebrow exposure, or any of the straightforward or extended endoscopic anterior skull base techniques. Although radiation therapy is much less often used as adjunctive treatment for pituitary tumors, many tumors, particularly those that are invasive of surrounding structures or recurrent, will eventually require this modality of treatment. The major advance has been the development of stereotactic radiosurgery and its application to suitable pituitary and parasellar tumors as both primary and adjuvant therapy. Further advances in all these treatment modalities will continue. Within our lifetime, the surgical treatment of pituitary lesions will become as foreign to us as our current techniques would have been to the forefathers of pituitary surgery. Versatility is one of the most important factors in the successful surgical management of sellar and parasellar pathology. A disease-oriented approach by a multispecialty team is the key to optimal management for patients with these difficult and challenging disorders. History of Transsphenoidal Surgery Gandhi CD, Christiano LD, Eloy JA, Prestigiacomo CJ, Post KD. The historical evolution of transsphenoidal surgery: facilitation by technological advances. Neurosurg Focus 2009;27(3):E8 Liu JK, Cohen-Gadol AA, Laws ER, et al. Harvey Cushing and Oskar Hirsch: early forefathers of modern transsphenoidal surgery. J Neurosurg 2005;103(6):1096–1104 Liu JK, Das K, Weiss MH, Laws ER, Couldwell WT. The history and evolution of transsphenoidal surgery. J Neurosurg 2001;95(6):1083–1096 History of Microneurosurgery Kriss TC, Kriss VM. History of the operating microscope: from magnifying glass to microneurosurgery. Neurosurgery 1998;42(4):899–907, discussion 907–908 History of Radiosurgery Laws ER, Vance ML. Radiosurgery for pituitary tumors and craniopharyngiomas. Neurosurg Clin N Am 1999;10(2):327–336 Lederman M. The early history of radiotherapy: 1895-1939. Int J Radiat Oncol Biol Phys 1981;7(5):639–648 History of Endoscopic Pituitary Surgery Pettorini BL, Tamburrini G. Two hundred years of endoscopic surgery: from Philipp Bozzini’s cystoscope to paediatric endoscopic neurosurgery. Childs Nerv Syst 2007;23(7):723–724 Prevedello DM, Doglietto F, Jane JA, et al. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007;107(1):206–213
 Early Approaches to the Sellar and Parasellar Region
Early Approaches to the Sellar and Parasellar Region
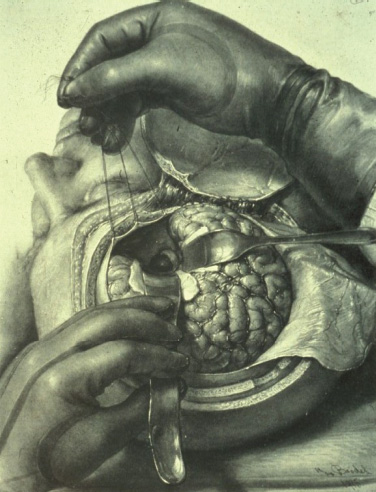
 The Birth of Microneurosurgery
The Birth of Microneurosurgery
 The Resurgence of Transsphenoidal Surgery
The Resurgence of Transsphenoidal Surgery
 Radiosurgery of the Pituitary and Surrounding Areas
Radiosurgery of the Pituitary and Surrounding Areas
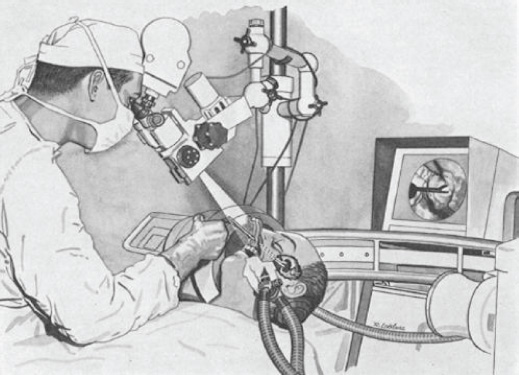
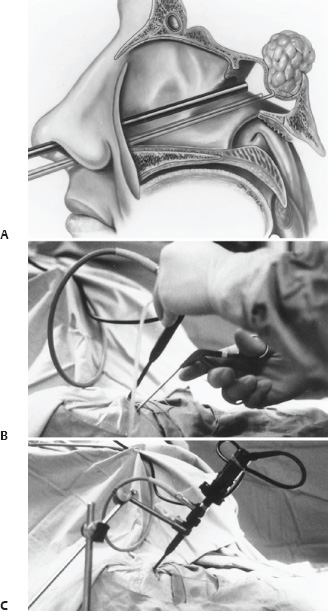
 The Rise of Endoscopic Skull Base Surgery
The Rise of Endoscopic Skull Base Surgery
 Conclusion
Conclusion
Suggested Readings
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree