Chapter 12A Language and Speech Disorders
Aphasia and Aphasic Syndromes
Language Disorders: Overview
Definitions
Aphasia is defined as a disorder of language acquired secondary to brain damage. This definition, adapted from Alexander and Benson (1997), separates aphasia from several related disorders. First, aphasia is distinguished from congenital or developmental language disorders, called dysphasias. (In contrast with British usage, in the United States the term dysphasia applies to developmental language disorders rather than partial or incomplete aphasia.)
Third, aphasia is distinguished from disorders of thought. Thought involves the mental processing of images, memories, and perceptions, usually but not necessarily involving language symbols. Psychiatric disorders derange thought and alter the content of speech without affecting its linguistic structure. Schizophrenic patients, for example, may manifest bizarre and individualistic word choices, with loose associations and a loss of organization in discourse together with vague or unclear references and communication failures (Docherty et al., 1996). Elementary language and articulation, however, are intact. Abnormal language content in psychiatric disorders is therefore not considered to represent aphasia, because the disorder is more one of thought than of language. Language disorders associated with diffuse brain diseases such as encephalopathies and dementias do qualify as aphasias, but the involvement of other cognitive functions distinguishes them from aphasia secondary to focal brain lesions.
Relevant Neuroanatomy
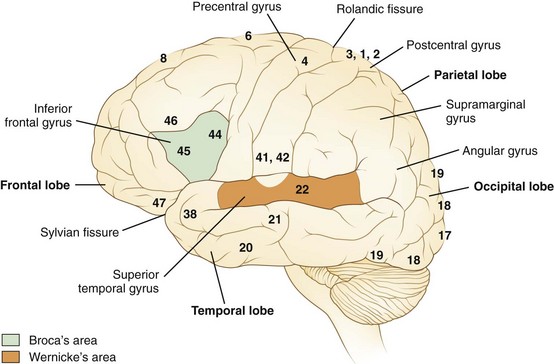
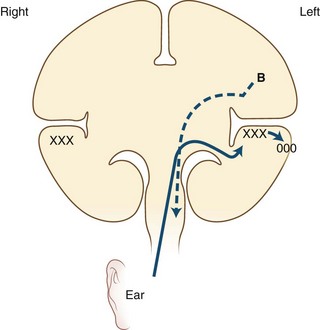
Language processes have a clear neuroanatomical basis. In simplest terms, the reception and processing of spoken language take place in the auditory system, beginning with the cochlea and proceeding through a series of way stations to the auditory cortex, the Heschl gyrus, in each superior temporal gyrus. Decoding sounds into linguistic information involves the posterior part of the left superior temporal gyrus, the Wernicke area or Brodmann area 22, which gives access to a network of cortical associations to assign word meanings. For both repetition and spontaneous speech, auditory information is transmitted to the Broca area in the posterior inferior frontal gyrus. This area of cortex “programs” the neurons in the adjacent motor cortex subserving the mouth and larynx, from which descending axons travel to the brainstem cranial nerve nuclei. The inferior parietal lobule, especially the supramarginal gyrus, also may be involved in phoneme processing in language comprehension and in phoneme production for repetition and speech (Hickok and Poeppel, 2000). These anatomical relationships are shown in Figs. 12A.1 and 12A.2. Reading requires perception of visual language stimuli by the occipital cortex, followed by processing into auditory language information via the heteromodal association cortex of the angular gyrus. Writing involves activation of motor neurons projecting to the arm and hand. A French study that used aphasia testing and magnetic resonance imaging (MRI) scans to evaluate 107 stroke patients confirmed the general themes of nearly 150 years of clinical aphasia research: that frontal lesions caused nonfluent aphasia, whereas posterior temporal lesions affected comprehension (Kreisler et al., 2000).
In right-handed people, and in a majority of left-handers as well, clinical syndromes of aphasia result from left hemisphere lesions. Rarely, aphasia may result from a right hemisphere lesion in a right-handed patient, a phenomenon called crossed aphasia (Bakar et al., 1996). In left-handed persons, language disorders are usually similar to those of right-handed persons with similar lesions, but occasional cases manifest with atypical syndromes that suggest a right hemisphere capability for at least some language functions. For example, a patient with a large left frontotemporoparietal lesion may have preserved comprehension, suggesting right hemisphere language comprehension. For the same reason, recovery from aphasia may be better in some left-handed than in right-handed patients with left hemisphere strokes.
Diagnostic Features
Muteness, a total loss of speech, may represent severe aphasia (see Aphemia later in the chapter). Muteness also can be a sign of dysarthria, frontal lobe dysfunction with akinetic mutism, severe extrapyramidal system dysfunction (as in Parkinson disease), non-neurological disorders of the larynx and pharynx, or even psychiatric syndromes such as catatonia. Caution must therefore be taken in diagnosing the mute patient as aphasic. A good rule of thumb is that if the patient can write or type and the language form and content appear normal, the disorder is probably not aphasic in origin. If the patient cannot speak or write but makes apparent effort to vocalize, and if there is also evidence of deficient comprehension, aphasic muteness is likely. Associated signs of a left hemisphere injury, such as right hemiparesis, also aid in diagnosis. Finally, if the patient gradually begins to make sounds containing paraphasic errors, aphasia can be identified with confidence.
Chronic encephalopathies, or dementias, pose a more difficult diagnostic problem because involvement of the language cortex produces readily detectable language deficits, especially involving naming, reading, and writing. These language disorders (see Language in Dementing Diseases later in this chapter) differ from aphasia secondary to focal lesions mainly by the involvement of other cognitive functions such as memory and visuospatial processes.
Bedside Language Examination
D. Frank Benson and Norman Geschwind popularized a bedside language examination of six parts, updated by Alexander and Benson (1997) (Box 12A.1). This examination provides useful localizing information about brain dysfunction and is well worth the few minutes it takes.
Auditory comprehension is tested first by asking the patient to follow a series of commands of one, two, and three steps. An example of a one-step command is “Stick out your tongue”; a two-step command is “Hold up your left thumb and close your eyes.” Successful following of commands ensures adequate comprehension, at least at this simple level, but failure to follow commands does not automatically establish a loss of comprehension. The patient must hear the command, understand the language the examiner speaks, and possess the motor ability to execute it, including the absence of apraxia. Apraxia (see Chapter 10 for full discussion) is defined operationally as the inability to carry out a motor command despite normal comprehension and normal ability to carry out the motor act in another context, such as for imitation or with use of a real object. Because apraxia is difficult to exclude with confidence, it is advisable to test comprehension by tasks that do not require a motor act, such as yes/no questions, or by commands that require only a pointing response. The responses to nonsense questions (e.g., “Do you vomit every day?”) quickly establish whether the patient comprehends. Nonsense questions often produce surprising results because of the tendency of some aphasics to cover up comprehension difficulty with social chatter.
Repetition of words and phrases should be deliberately tested. Dysarthric patients and those with apraxia of speech (see Chapter 12B) have difficulty with rapid sequences of consonants, such as in “Methodist Episcopal,” whereas aphasic persons have special difficulty with grammatically complex sentences. The phrase “no ifs, ands, or buts” is especially challenging for aphasics. Often, aphasics can repeat familiar or “high-probability” phrases much better than unfamiliar ones.
Aphasic Syndromes
Broca Aphasia
In 1861, the French physician Paul Broca described two patients, establishing the aphasia syndrome that now bears his name. The speech pattern is nonfluent; on bedside examination, the patient speaks hesitantly, often producing the principal meaning-containing nouns and verbs but omitting small grammatical words and morphemes. This pattern is called agrammatism or “telegraphic speech.” An example is “wife come hospital.” Patients with acute Broca aphasia may be mute or may produce only single words, often with dysarthria and apraxia of speech. They make many phonemic errors, inconsistent from utterance to utterance, with substitution of phonemes usually differing only slightly from the correct target (e.g., /p/ for /b/). Naming is deficient, but the patient often manifests a “tip-of-the-tongue” phenomenon, getting out the first letter or phoneme of the correct name. Paraphasic errors in naming more frequently are of literal than of verbal type. Auditory comprehension seems intact, but detailed testing usually reveals some deficiency, particularly in the comprehension of complex syntax. For example, for persons with Broca aphasia, sentences with embedded clauses involving prepositional relationships cause difficulty in comprehension as well as in expression (“The rug that Bill gave to Betty tripped the visitor”). A positron emission tomography (PET) study in normal persons (Caplan et al., 1998) showed activation of the Broca area in the frontal cortex during tests of syntactic comprehension; the Broca area thus appears to be involved in syntactical operations, both expressively and receptively. Repetition is hesitant in these patients, resembling their spontaneous speech. Reading often is impaired despite relatively preserved auditory comprehension. Benson termed this reading difficulty of Broca aphasics the “third alexia,” in contradistinction to the two classical types of alexia (see Aphasic Alexia later in the chapter). Patients with Broca aphasia may have difficulty with syntax in reading, just as in auditory comprehension and speech. Writing is virtually always deficient in Broca aphasics. Most patients have a right hemiparesis necessitating use of the nondominant left hand for writing, but this left-handed writing is far more abnormal than the awkward renditions of a normal right-handed person attempting to write left-handed. Many patients can scrawl only a few letters.
Associated neurological deficits of Broca aphasia include right hemiparesis, hemisensory loss, and apraxia of the oral apparatus and the nonparalyzed left limbs. Apraxia in response to motor commands is important to recognize because it may be mistaken for comprehension disturbance. As mentioned earlier, comprehension should also be tested by responses to yes/no questions or commands to point to an object. The common features of Broca aphasia are listed in Table 12A.1.
Table 12A.1 Bedside Features of Broca Aphasia
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Nonfluent, mute or telegraphic, usually dysarthric |
| Naming | Impaired |
| Comprehension | Intact (mild difficulty with complex grammatical phrases) |
| Repetition | Impaired |
| Reading | Often impaired (“third alexia”) |
| Writing | Impaired (dysmorphic, dysgrammatical) |
| Associated signs | Right hemiparesis |
| Right hemisensory loss | |
| ± Apraxia of left limbs |
An important clinical feature of Broca aphasia is its frequent association with depression (Robinson, 1997). Patients with Broca aphasia typically are aware of and frustrated by their deficits. At times they become withdrawn and refuse help or therapy. Usually the depression lifts with recovery from the deficit, but it may be a limiting factor in rehabilitation.
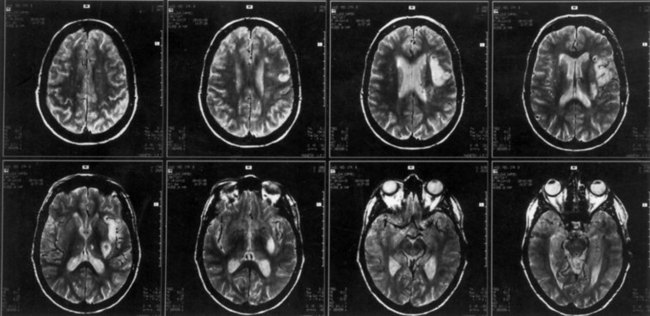
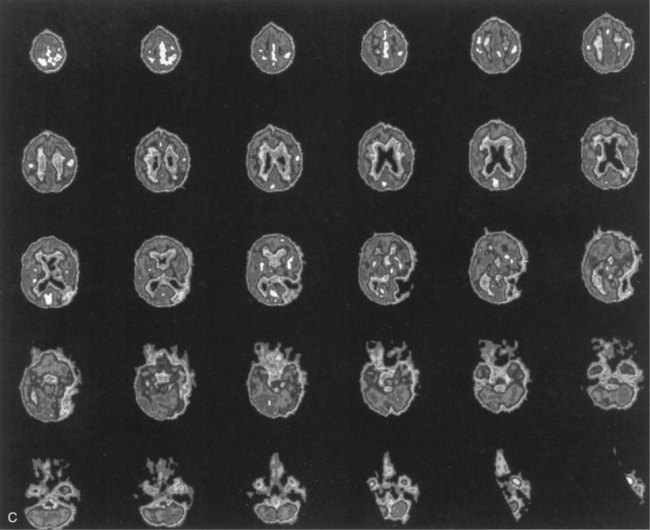
The lesions responsible for Broca aphasia usually include the traditional Broca area in the posterior part of the inferior frontal gyrus, along with damage to adjacent cortex and subcortical white matter. Most patients with lasting Broca aphasia, including Broca’s original cases, have much larger left frontoparietal lesions, including most of the territory of the upper division of the left middle cerebral artery. In such patients, the deficit typically evolves from global to Broca aphasia over weeks to months. Patients who manifest Broca aphasia immediately after their strokes, by contrast, have smaller lesions of the inferior frontal region, and their deficits generally resolve quickly. In computed tomography (CT) scan analyses at the Boston Veterans Administration Medical Center, lesions restricted to the lower precentral gyrus produced only dysarthria and mild expressive disturbance. Lesions involving the traditional Broca area (Brodmann areas 44 and 45) resulted in difficulty initiating speech, and lesions combining the Broca area, the lower precentral gyrus, and subcortical white matter yielded the full syndrome of Broca aphasia. In other studies at the center, damage to two subcortical white matter sites—the rostral subcallosal fasciculus deep to the Broca area and the periventricular white matter adjacent to the body of the left lateral ventricle—was required to cause permanent nonfluency. These concepts concerning the Broca area and its mainly temporary role in Broca aphasia have been confirmed by a recent MRI study, indicating that MRI lesions in the Broca area correlate with Broca or global aphasia in acute stroke, but not in the chronic period (Ochfeld et al., 2010). Fig. 12A.3 shows an MRI scan of the brain from a patient with Broca aphasia.
Wernicke Aphasia
Associated signs are limited in Wernicke aphasia; most patients have no elementary motor or sensory deficits, although a partial or complete right homonymous hemianopia may be present. The characteristic bedside examination findings in Wernicke aphasia are summarized in Table 12A.2.
Table 12A.2 Bedside Features of Wernicke Aphasia
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Fluent with paraphasic errors; usually not dysarthric, sometimes logorrheic |
| Naming | Impaired (often bizarre paraphasic misnaming) |
| Comprehension | Impaired |
| Repetition | Impaired |
| Reading | Impaired for comprehension, reading aloud |
| Writing | Well formed, paragraphic |
| Associated signs | ± Right hemianopia |
| Motor, sensory signs usually absent |
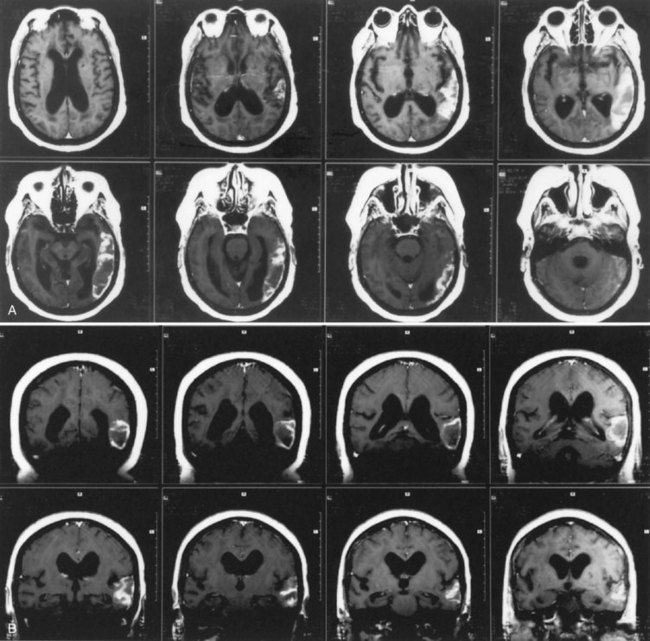
The lesions of patients with Wernicke aphasia usually involve the posterior portion of the superior temporal gyrus, sometimes extending into the inferior parietal lobule. Fig. 12A.4 shows a typical example. The exact confines of the Wernicke area have been much debated. Damage to this area (Brodmann area 22) has been reported to correlate most closely with persistent loss of comprehension of single words, although only larger temporoparietal lesions have been found in patients with lasting Wernicke aphasia. In the acute phase, the ability to match a spoken word to a picture is quantitatively related to decreased perfusion of the Wernicke area on perfusion-weighted MRI, indicating less variability during the acute phase than after recovery has taken place (Hillis et al., 2001). Electrical stimulation of the Wernicke area produces consistent interruption of auditory comprehension, supporting the importance of this region for decoding auditory language. A receptive speech area in the left inferior temporal gyrus has also been suggested by electrical stimulation studies and by a few descriptions of patients with seizures involving this area (Kirshner et al., 1995), but aphasia has not been recognized with destructive lesions of this area. Extension of the lesion of Wernicke aphasia into the inferior parietal region may predict greater involvement of reading comprehension. In terms of vascular anatomy, the Wernicke area lies within the territory of the inferior division of the left middle cerebral artery.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree