Chapter 189 Management of Cauda Equina Tumors
Most of these tumors are benign, which justifies an aggressive surgical approach, using magnification techniques and microsurgical instrumentation. Adjuvant therapy is unnecessary, at least after the first procedure. In 1996 we performed a retrospective review of the French cases with 231 patients.1,2 Our personal series comprises 31 patients. It is not always easy to make a distinction between a true CET and a tumor arising from the neighboring structures, even with the magnetic resonance imaging (MRI) data, and it is sometimes necessary to obtain the operative and anatomopathologic findings to correctly classify the tumor. In the literature, many isolated cases are reported, but large series and practical advice for management are seldom encountered.
Definition of Cauda Equina Tumors
Primary tumors of the cauda equina arise from the different intrinsic structures of the region, such as the filum, the nerve sheaths, intrinsic vessels of the nerves, conjunctive tissue, and embryologic remnants.3 Some authors include tumors sprouting from the surrounding tissues—meningeal envelopes, epidural structures, and bone—but this does not seem to be the proper nosologic approach in description of this pathology, even if their clinical features present some similarities. It is in fact relatively difficult to classify metastatic tumors that, although not infrequent, do not constitute a surgical problem (Fig. 189-1).
Clinical Presentation
The involved population includes both sexes, with a slight male predominance, which is more marked for ependymomas (nearly 61%).1,2 Mean age for all CETs is 47 years, but there is a strong correlation between age and tumor type: 34.6 years ± 16 years for ependymomas and 51 years ± 17 years for benign neurinomas.
The time interval between the first symptom and the diagnosis ranges from 1 month to 264 months. In our series the mean delay was about 34 months for 50% of the patients. There is no influence of age but rather a strong correlation with tumor type: long-lasting time lapse, 50 months for neurofibromas and paragangliomas; medium, 20 months for neurinomas and ependymomas; short for malignant tumors, especially metastasis. In a few cases intratumoral bleeding caused by a trauma results in an acute cauda equina syndrome.4
Diagnostic Evaluation
Electromyography and evoked potentials are of limited value in the investigation of CETs.
Magnetic Resonance Imaging
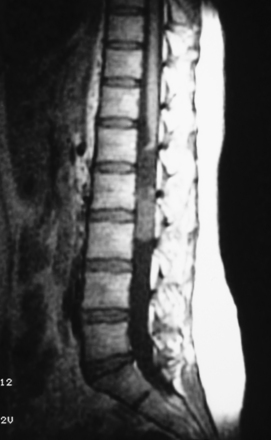
MRI is the gold standard for diagnosis. It must include T1- and T2-weighted sequences, without and with gadolinium administration, and in sagittal and axial views. It demonstrates the existence and the size of the tumor, cystic components, intratumor bleeding, vascularity, and the relation of the tumor to the conus, and it is contributive in 100% of cases. It also rules out other pathologies. It leads to a presumptive histopathologic diagnosis.5
Management Decisions
The functional problems and prognosis have to be precisely described to the patient and his or her family, and the expectations must be realistic. Patients harboring minor neurologic impairment must be aware that neurologic deterioration can result from the operation, and in all cases hospitalization in a rehabilitation center will probably be necessary. The sphincter dysfunction is the least likely symptom to improve after surgery if it has existed for several weeks, and it may be amplified or appear postoperatively. As in intramedullary spinal tumors, the final outcome depends on the preoperative neurologic status of the patient. Because those tumors are nearly always benign, there is no alternative treatment to surgery. The goals of the operation are tumor removal and preservation or improvement of neurologic function.
Technical Adjuncts to Tumor Removal
Intraoperative stimulation of motor roots may be used for their identification when they are surrounded by tumor tissue.6 Evoked potential recordings are sometimes used, but they also are far less effective than in spinal cord tumors, and there is no evidence that they improve outcome.
Surgical Technique
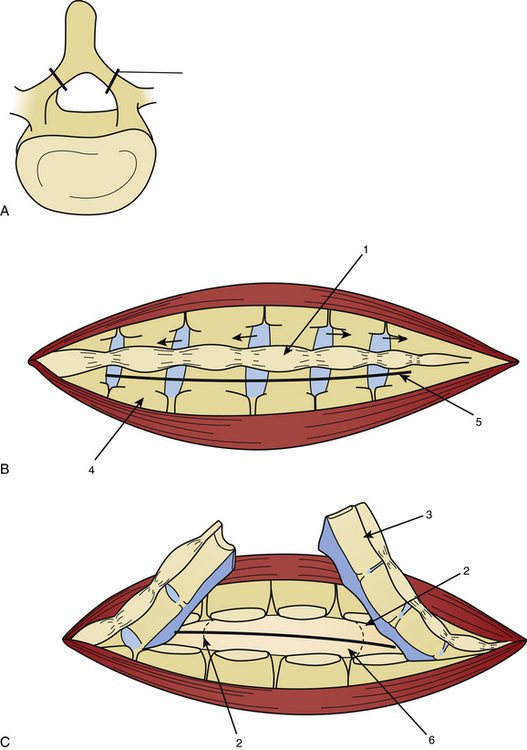
Laminotomy must be performed systematically in infants and young adults, with reconstruction of a normal anatomy by reinserting the posterior processes prevents growth deformation in the young but also decreases the post-operative pain and the frequency of CSF leaks (Fig. 189-2).
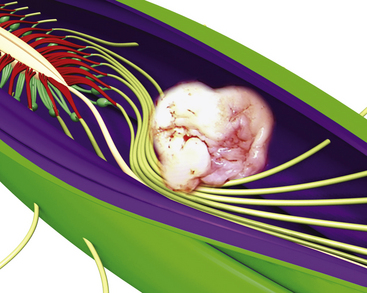
The dura mater may be absent in large ependymomas, and care must be taken not to injure the roots during the laminotomy. In the event of reoperation, laminectomy is unavoidable. The dura opening must allow control of the tumor’s extremities (Fig. 189-3), and the dura is fixed with stitches to the muscles. Dividing the tumor under optic magnification may begin. The best way to proceed is to start at the upper limit of the tumor, proceed inferiorly to the lower (Fig. 189-4), and to finish at the central part of the tumor. This strategy allows optimal root control (Fig. 189-5). Most of the roots are pushed against the dura by the tumor. Tumors of the filum terminale displace the roots on the right and left sides of the spinal canal, but they can develop between the roots, covering and hiding them. It is important to identify the filum, and stimulation can help do this.
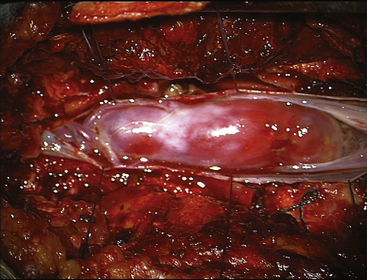
FIGURE 189-3 Operative view showing exposure of both extremities of a cauda equina paraganglioma after opening of the dura mater.
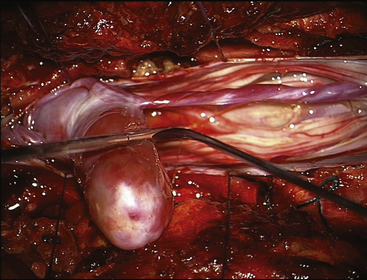
FIGURE 189-4 Operative view of the same patient as Figure 189-3 showing debulking of the superior pole of the tumor.
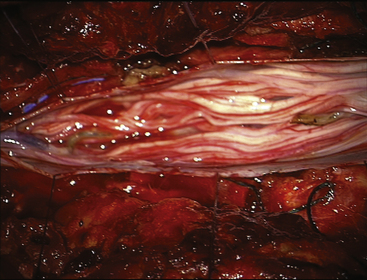
FIGURE 189-5 Operative view of the same patient as in Figures 189-3 and 189-4 demonstrating root preservation after tumor removal.
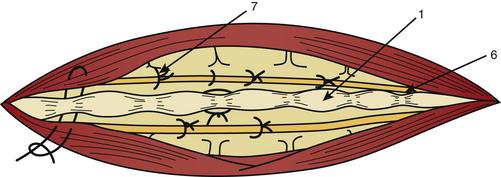
After complete tumor removal and careful hemostasis, the dura is closed. A dural graft is often necessary.7 The spine is reconstructed and the lumbar aponeurosis is attached to the spinous processes. It is recommended that one leave some aponeurosis attachment during the opening to facilitate this type of closure (Fig. 189-6). The subcutaneous fat and skin are closed as usual. The use of tissue glue is left to the preference of the surgeon. All the removed material is sent for histopathologic examination.
Histologic Findings and their Consequences
Adult Pathology
Neurinomas (Schwannomas)
Neurinomas can develop purely inside the spinal canal or simultaneously in the foramen (hourglass type), which can cause an enlargement of the neural foramen seen on neuroimaging. On CT scan and MRI there is homogeneous enhancement (Figs. 189-7 and 189-8). The tumor is well encapsulated, and complete removal can be achieved in most cases. Magnification allows identification of the rootlet bearing the tumor and preservation of the other roots (Fig. 189-9). The bearing rootlet is cut 1 cm above and 1 cm below the tumor, some very small satellite tumors or tumor prolongments being at times in close proximity to the tumor.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree