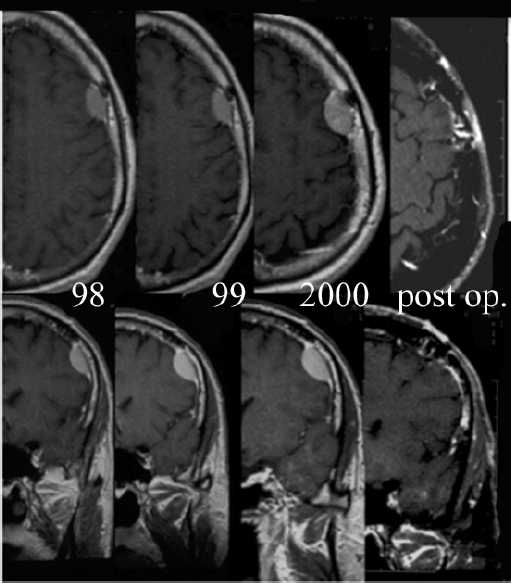
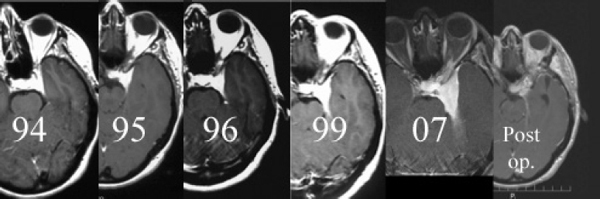
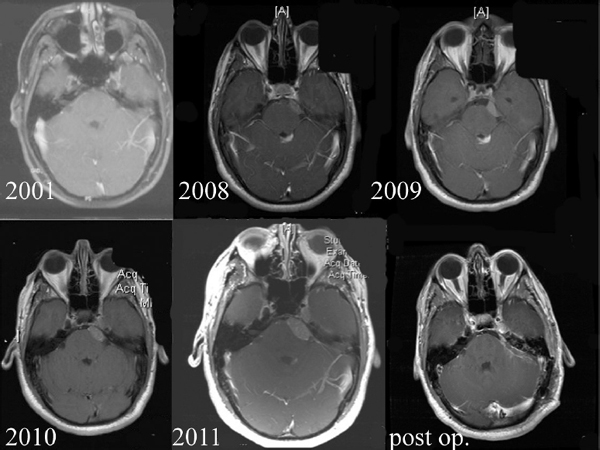
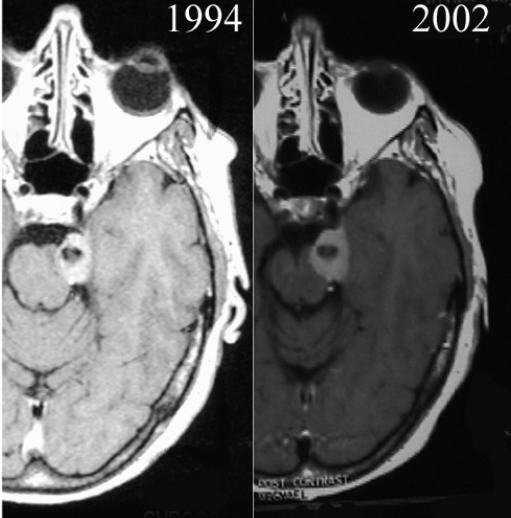
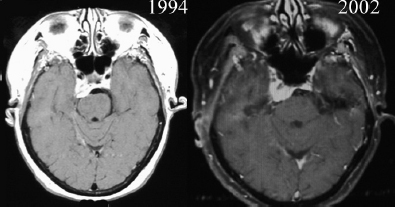
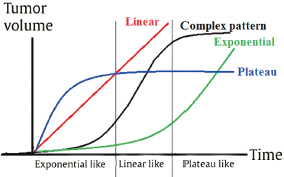
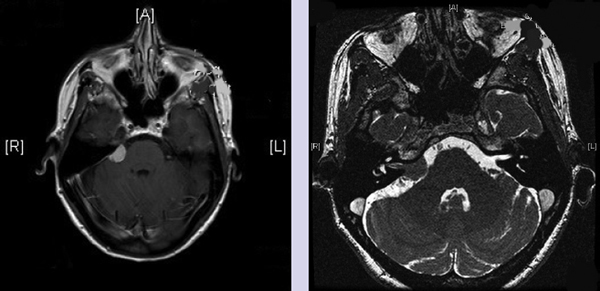
Chapter 4 Case A 39-year-old woman came to medical attention with headache. The magnetic resonance images show a meningioma of 1.5 cm in the maximum diameter. Participants Observation of Incidental Meningiomas: Mohamad Abolfotoh and Ossama Al-Mefty Stereotactic Radiosurgery for Small Meningiomas: Toshinori Hasegawa The Role of Microsurgery in the Management of Small Skull-Base Meningiomas: Luis A.B. Borba and Daniel D. Cavalcanti Moderator: Management of Incidental Meningiomas: E. Antonio Chiocca The magnetic resonance imaging (MRI) study of the patient presented shows a small enhanced lesion in the cerebellopontine angle (CPA) cistern measuring 10.5 × 16 mm in axial views. This lesion is dural based and isointense on T2-weighted images, excluding the possibility of a vestibular schwannoma or any of the rapidly growing lesions that might mimic a meningioma. According to the available clinical and radiological data, this lesion is likely to be an incidentally discovered CPA meningioma. Although the exact definition of (asymptomatic) incidentally discovered meningiomas (IDMs) remains unclear,1 the term refers to meningiomas discovered during a brain workup for any reason other than a known specific clinical presentation of a detected tumor,2–5 related to neurofibromatosis type 2, present as a part of multiple meningiomas, or induced by radiation.5 The rate of incidence of asymptomatic meningiomas has increased in the past decade, clearly because of the availability of, and advances in, diagnostic techniques.1,6 These meningiomas now account for almost half of diagnosed meningiomas.5 As this patient had only a headache, we could not immediately determine that this small lesion was the cause of her symptoms. Headache is a common presentation for relatively large CPA meningiomas7 but is uncommon for smaller, premeatal, or intracanalicular ones.8,9 Headache has never been postulated to be the specific or the main presentation for small CPA meningiomas.7–9 The meningioma in the images appears to be a premeatal type of CPA meningioma, which is nearly always discovered early and is consequently small at the time of diagnosis. It does not commonly present with headache, but instead with symptoms of fifth, seventh, and eighth cranial nerve involvement.7,10,11 Headache and dizziness are the most common reasons for brain imaging that leads to the discovery of IDMs.2–4 Many authors investigating IDMs believe them unlikely to cause headache, as these tumors are always small without causing mass effect.2–4,12 Thus, for this patient, our approach would be based on the diagnosis of an IDM. With the exception of IDMs in certain locations such as the tuberculum sellae, we recommend conservative follow-up for asymptomatic IDMs, especially many skull-base IDMs, as some reports indicate that most are World Health Organization (WHO) grade I and are indolent in nature.13–15 This recommendation is based on experience and a review of the literature. To the best of our knowledge, studies of IDMs overwhelmingly have recommended conservative close follow-up.1–3,12,16–22 Recent criteria for managing IDMs have suggested observation for asymptomatic lesions in patients regardless of age, but for growing lesions in patients age 65 years or younger, surgery is recommended regardless of the size of the tumor.6 The rationale for conservative treatment of IDMs is that it defers the risks of intervention in asymptomatic patients.23 However, although the risk is lower in young patients with relatively small lesions, like the patient described here, it is still not negligible. Nakamura and colleagues3 concluded that most asymptomatic meningiomas show only minimal growth and may be observed without any surgical intervention, but close observation is recommended in young patients because of the higher tumor growth rates in this age group. Conservative management (observation) is the initial treatment option for elderly patients, for medically unstable patients with small tumors and mild, stable symptoms, and for those who are unwilling to undergo surgery.7 The dilemma for neurosurgeons is the treatment of young people who are medically fit for surgery. Asymptomatic young persons (such as the present patient) can be followed, with intervention necessary only when symptoms appear or when progressive growth is documented. The radiological diagnosis of meningiomas is usually straightforward. Other more aggressive lesions can mimic meningiomas, however, so close clinical and radiological follow-up is mandatory when giving the patient the conservative option of observation.4,6 Once a meningioma is discovered, we schedule follow-up scans at 3 and 9 months, and then yearly or perhaps every other year if the patient’s condition is stable for 5 years. The importance of the early first MRI (after 3 months) is to exclude rapidly enlarging meningiomas or any other aggressive lesion that may mimic a meningioma. This follow-up protocol is more or less similar to other protocols reported in the literature.4,16 It is critical to compare the last study to the previous ones, particularly the original or even an earlier prediagnostic study. Comparing the latest study to only the preceding one might be misleading and could miss significant changes that have taken place over time. During the follow-up period, if the lesion has begun to enlarge but is still asymptomatic, we do an MRI study every 6 months; if the lesion shows significant progressive growth, we proceed with surgical treatment (Fig. 4.1). At any time during the period of observation, if specific symptoms appear (Fig. 4.2) or progressive growth is documented (Fig. 4.3), we prefer to proceed with surgery, but many untreated lesions might show no growth over a long period of time (Figs. 4.4, 4.5). Fig. 4.1 Enhanced serial axial and coronal magnetic resonance imaging (MRI) studies of a 50-year-old man showing the progressive growth of a left frontal incidentally discovered meningioma (IDM) over 2 years. This progression led to the decision for surgical excision. Before treating IDMs, one should understand their natural history and rate of growth, along with other factors that might influence the decisions regarding management. We classify factors affecting the management of IDMs into three groups: Fig. 4.2 Enhanced serial axial MRI studies of a meningioma in the left cavernous sinus of a 42-year-old woman show progressive growth of the tumor, which became symptomatic and has been operated on. 1. Patient factors: the patient’s age, overall health, and fitness for surgery 2. Tumor factors: the location, size at diagnosis, radiological aspects, natural history, and rate of growth 3. Circumstances: the experience of the neurosurgeon and the availability of treatment facilities, which increase the chance of a good outcome and minimize morbidity. Nonetheless, the management of IDMs should be determined on an individual basis, case by case, because all these factors must be considered in the decision-making process.17 The age of the patient is the most important factor because the growth rate of IDMs in the elderly is very slow.1,3,4,6,16–19 Rubin and colleagues12 found that older age was significantly related to a low rate of tumor growth; every additional year in the patient’s age decreases the risk of tumor growth by 8%, a fact that agrees with most previous studies.1,3,18,20 In addition, the risks associated with surgery are much greater in older patients. Awad and associates24 reported on 75 patients older than 60 years who underwent surgical resection of intracranial meningiomas, 21% of which were asymptomatic. Perioperative morbidity was 48% and operative mortality was 6.6%. Thus, there is agreement among neurosurgeons that conservative management is the first choice of treatment for elderly patients with IDMs that are asymptomatic or even those that cause mild, stable symptoms.6,7,18 The debate is about how to manage asymptomatic IDMs in younger patients, as it is now well known that higher growth rates of meningiomas are associated with the patient’s young age.5,6,12,16 Nevertheless, for us, this fact is not sufficient to warrant radiation or surgery. Understanding the natural history of the growth of IDMs is key to making the decision. In young people with asymptomatic lesions, although the risks associated with surgery are fewer than in the elderly, conservative management is still our initial choice, as IDMs rarely become symptomatic within a follow-up period of 2 years or less.5 Many authors have found observation acceptable if a patient has an asymptomatic IDM, is under the age of 60, and is reluctant to undergo surgery or is not in optimal medical condition, as even tumor growth is usually not associated with morbidity.2,4,12,18,25 Fig. 4.4 Enhanced axial MRI studies of a small IDM in a 63-year-old woman. The tumor relates to the petrous apex and has a large central area of calcification. It showed no growth over a period of 8 years. Before the turn of the 21st century, we knew little about the natural history of IDMs. Now the picture is getting clearer (Table 4.1), but the definition and methods of calculating tumor growth still vary. In volume measurement studies, an annual growth rate of more than 1 cm3 per year or a volume increase of greater than 15% are considered to indicate tumor growth. In diameter measurement studies, growth has been defined as a change in tumor size of 2 or 5 mm, or even any measurable change.5 In early studies, a large diameter was used to determine tumor size. This method may be useful for judging the treatment response of gliomas to adjuvant therapy, but it is not accurate for evaluating the exact increase in the volume of skull-base meningiomas because of their complex shape; these tumors may grow in any direction.1,4,18 Volumetry appears to reflect the tumor’s size more accurately than does measuring the diameter because it is applicable to irregularly shaped tumors. For that reason, it has been used in recent studies to evaluate lesion size.1,3,16,21,26 In practice, we still usually rely on measuring the maximum tumor diameter in two directions. This method is practical and less time-consuming than volumetry in the clinical setting; we also believe that a very small increase (even in diameter) can cause symptoms and may change the treatment options. Fig. 4.5 Enhanced axial MRI studies of a right posterior cavernous IDM in a 68-year-old woman. The tumor showed no growth over a period of 8 years. Herscovici and associates17 found that 32 of 51 IDMs showed no growth at the end of follow-up (mean 67 months). However, the percentage of patients who showed tumor growth by the end of follow-up ranges from 0 to 44% across the literature (Table 4.1).27,28 The IDMs do not always follow an exponential growth pattern, but always exhibit complex patterns of growth (Fig. 4.6). Nakasu and associates26 reported that benign meningiomas grow exponentially, linearly, or not at all. Hashiba and colleagues1 noticed similar results in their large series of IDMs, also suggesting that some tumors show a complex pattern of growth throughout follow-up. Thus, a tumor might grow exponentially in its early stages, linearly in the intermediate stage, and finally reach a plateau at the terminal stage (Fig. 4.6). This argument means that the tumor doubling time or annual growth rate calculated by observation over a short period of time may not predict the growth potential and natural history of a tumor over the life of the patient.1,22 For this reason, we do not start intervention for asymptomatic IDMs until after the appearance of new, specific symptoms or if the tumor shows a progressive and significant increase in size on serial follow-up scans. In the published literature, neither the patient’s sex nor the tumor location was found to predict the growth of an IDM; however, it is well known that skull-base IDMs grow slowly. Van Havenbergh and colleagues19 retrospectively studied the natural history of 21 conservatively treated petroclival meningiomas and indeed found them to be slowly growing tumors. Growth rates were higher among patients with small meningiomas, although the growth index was not significantly correlated with the tumor’s diameter or volume at the time of diagnosis. The site of the tumor is important in the decision for intervention. For example, tuberculum sellae meningiomas are known to be associated with visual deterioration within a relatively short time, even when the tumor is less than 2 cm,5 which justifies resection at their discovery. The surgical removal of a convexity meningioma is associated with minimal risk, which indicates the need to proceed with surgical excision at discovery or whenever the meningioma shows enlargement in young patients (Fig. 4.1). A concern over the probable aggressive nature of falcine meningiomas (particularly in males) is also indicative of early intervention. The predictive value of a larger tumor size at diagnosis remains controversial.12,19 Nakamura and associates3 concluded that the initial tumor size is not a predictive factor for tumor growth, but the tumor’s size at presentation is considered a risk factor for the development of new symptoms, as meningiomas smaller than 2.5 cm usually remain silent during the next 5 years of follow-up.12,29 Fig. 4.6 A simple schematic diagram showing the different growth patterns of meningiomas: linear growth (red line), plateau (blue line), exponential (green line), and complex pattern (black line). (Adapted from Hashiba T, Hashimoto N, Izumoto S, et al. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg 2009;110:675-684. Reprinted by permission.) Calcification may be the most significant predictive factor of slow tumor growth.5 Many authors have pointed out that meningiomas without calcification on imaging are more likely to progress than are calcified meningiomas.1,3,18,20,25,26 Herscovici and colleagues17 found that 10 out of 28 nongrowing tumors had calcification. Thus, calcification allows a more conservative approach12 (Fig. 4.4). Oya and coworkers16 analyzed data on 244 patients with 273 conservatively treated tumors; their results suggest that hyperintensity on T2-weighted MRI is associated with a higher rate of tumor growth. The question is, Why do we not recommend primary radiosurgery for such tumors? In reviewing the literature, we found that the control rate of meningiomas after stereo-tactic radiosurgery does not look much different from the natural history of untreated IDMs. Moreover, the growth pattern of such meningiomas after failed control with radiosurgery is worse than the natural history of untreated meningiomas.30 It has been confirmed that the long-term follow-up of radiosurgery shows a continuous decline in control rates over time. A study of the Kaplan-Meier curve provided in the large series of radiosurgery for posterior fossa meningiomas by Starke and associates31 demonstrates that the control rate is 50% or lower by 15 years. A retrospective study comparing the Sheffield, England, database of radiosurgery-treated patients with national mortality and cancer registries showed an actuarial mortality of 47% at 15 years among patients treated with the gamma knife, with the progression of the meningioma listed as the cause of death in 69% of patients.32 These results indicate that the effect of radiosurgery at best (the term effect) is x number of years of delaying the progression of these meningiomas. Kondziolka and colleagues33 have one of the largest series of stereotactic treatment of meningiomas over a period of 20 years. They treated younger patients with minimal symptoms and those who were asymptomatic but decided against observation. Unfortunately, the outcomes in these two groups were not separately identified. In our experience, surgery after failed radiosurgery is not as straightforward as surgery on untreated tumors, but confirmatory reports are not found in the literature. In our practice, surgery after radiation is always associated with a higher risk of complications (especially cranial nerve palsies) and the lower success of tumor resection because of loss of the arachnoidal plane and adhesions to vital structures. So we recommend observing IDMs until they need intervention, at which point we recommend surgical resection if the patient is in good medical condition and has agreed to surgery after discussing other options. Based both on an intensive review of the literature and on our own experience, we recommend beginning with conservative management for patients with asymptomatic IDMs, with some exceptions. Because radiosurgery is not without risks (including associated morbidity), and its benefits are time-limited, we are not in favor of the primary treatment of IDMs with radiosurgery when progression has not been documented. The presented case is that of a 39-year-old woman with headache, indicating an incidentally found small petrous meningioma. The treatment strategy for this case includes three options: wait-and-see, microsurgery, and stereotactic radiosurgery. The aim of treatment is not to eliminate this tumor but to achieve tumor control during her lifetime without any neurologic deficits. Because this patient has no neurologic deficit at presentation, the wait-and-see strategy with periodic radiological studies can be a reasonable treatment option. When the tumor shows growth on the follow-up studies or causes neurologic deficits, it should be treated through either a craniotomy or radiosurgery. Jo and colleagues34 showed that 24 of 77 patients (31.2%) with asymptomatic meningiomas had their tumors progress during a mean follow-up period of 5 years. In their series, the 5-year actuarial tumor progression rate was 38%. Van Havenbergh and colleagues35 also reported the natural history of 21 patients with petroclival meningiomas, observing that 76% of patients had tumor growth during the follow-up period of 48 to 120 months, and 63% of the growing tumors caused functional deterioration. Considering the patient’s age and the natural history of meningiomas, early intervention may be recommended because the risk of morbidity after treatment is considered higher in patients with symptomatic meningiomas than in those with asymptomatic ones. Thus, to avoid morbidity either microsurgery or radiosurgery should be done before the progressive infiltration of cranial nerves or major vessels. Surgical resection is the most common treatment for young patients harboring meningiomas. The recent results of surgical resection have improved with the introduction of microsurgery, refinements in microsurgical techniques, and the use of navigation and monitoring systems, in addition to the development of various neuroimaging modalities, such as MRI, since Simpson36 published the postoperative recurrence rates of intracranial meningiomas in 1957. Most recently, Sughrue and associates37 documented the results of 373 patients who underwent initial resection for meningiomas of WHO grade I. With modern microsurgical techniques, the 5-year recurrence and progression-free survival was 95%, 85%, 88%, and 81% for patients achieving Simpson grades I, II, III, and IV resections, respectively, with a median follow-up of 3.7 years. Even patients who underwent Simpson grade I resection by experienced neurosurgeons have a 5-year recurrence risk of 5%. The duration of follow-up is too short to evaluate the true recurrence rate of benign meningiomas in this series, and the recurrence and progression rates are actually predicted to increase over time. The advantage of surgical resection is the ability to confirm the lesion with histological examination. In recent decades, stereotactic radiosurgery has emerged as a minimally invasive treatment for patients with intracranial lesions. Pollock and colleagues38 compared the tumor control between surgical resection (n = 136) and stereotactic radiosurgery (n = 62) in 198 patients with small to medium-sized benign meningiomas. The progression-free survival after radiosurgery was equivalent to that after Simpson grade I resection, whereas radiosurgery provided higher progression-free survival than Simpson grade II, III, and IV resections. Furthermore, additional treatments were more commonly required in patients who underwent surgical resection. Contrary to the complication rate of 10% in patients treated with radiosurgery, 22% of those who underwent surgical resection developed some kind of complications.
Management of
Incidental Meningiomas
Observation of Incidental Meningiomas
Images of the Presented Case
Incidentally Discovered Meningiomas
Clinical Picture of the Presented Case
Conservative Treatment
Follow-Up Protocol
Discussion
Conclusion
Stereotactic Radiosurgery for Small Meningiomas
Treatment Options
The Wait-and-See Strategy
Microsurgery
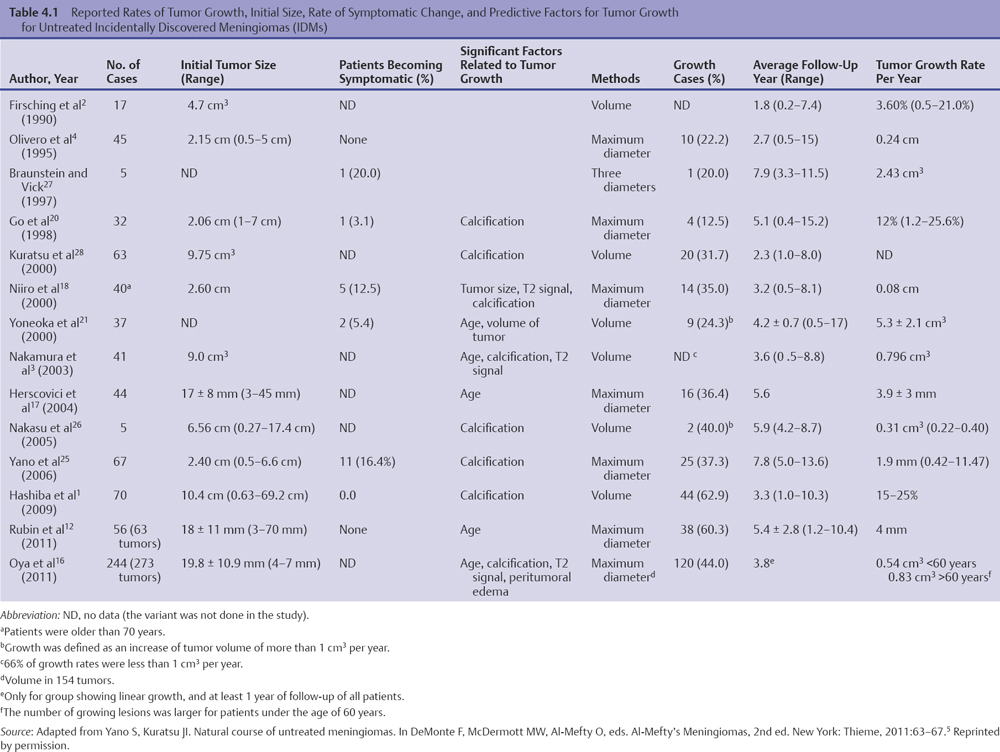
Variables | No. | % |
Age (years) | Range 15–91 | Median 54 |
Men/women | 41:108 | 28: 72 |
No. of prior surgeries | ||
0 | 70 | 47 |
1 | 52 | 35 |
2 | 16 | 11 |
≥ 3 | 11 | 7 |
Follow-up (months) | Range 3–193 | Median 126 |
≥ 5 years | 106 | 74 |
≥ 10 years | 82 | 57 |
Stereotactic Radiosurgery: Our Experience
Between May 1991 and April 1997, a consecutive 149 patients with meningiomas, excluding atypical or anaplastic meningiomas, were treated with gamma knife radiosurgery (GKRS) at our institute. Of these, nine patients had multiple lesions. A total of 162 lesions were treated. Five patients (five lesions) were lost to follow-up. The patients’ characteristics are shown in Table 4.2. Seventy patients (47%) underwent GKRS as the initial treatment, and the disease was diagnosed on the basis of neuroimaging findings alone. Tumor locations are shown in Table 4.3, and tumor sizes and irradiation doses at the time of treatment are shown in Table 4.4. The median follow-up period was 126 months (range 3–193 months). During the follow-up period, 17 patients died—five from meningioma progression, and 12 from other diseases.
The 10-year survival rate to death from a brain tumor after GKRS was 95%. A total of 157 lesions were evaluated with follow-up imaging studies. In-field or out-of-field treatment failure was found in 29 lesions. The actuarial 3-, 5-, and 10-year progression-free survival rates were 96%, 89%, and 77%, respectively. A multivariate analysis of factors affecting progression-free survival showed that infratentorial lesions were successfully treated significantly (p = 0.045). Of the 157 lesions, 19 had in-field treatment failure, including two lesions with symptomatic peritumoral edema, indicating that the actuarial 3-, 5-, and 10-year local tumor control rates were 98%, 93%, and 84%, respectively. Five patients experienced adverse radiation effects, two of whom had severe perifocal edema. Both of them had large supratentorial meningiomas with a tumor volume of 28 cm3 and 50 cm3. Other complications included oculomotor palsy in one patient with a cavernous sinus lesion, facial numbness in one with a CPA lesion, and a transient visual defect in one with a cavernous sinus lesion. In our experience, none of 29 patients with CPA meningiomas, like the patient described here, experienced treatment failure. In addition, if the results are limited to those whose tumors were 2 cm or less in mean diameter and who underwent initial treatment, 27 of 28 patients (96%) achieved good tumor control with a median follow-up of 130 months. Only one patient with a cavernous sinus lesion experienced in-field treatment failure at 70 months, requiring a repeated craniotomy and radiosurgery. These results encourage us to use stereo-tactic radiosurgery as the initial treatment for young patients with incidentally found meningiomas.
Location | No. | % |
Parasagittal | 8 | 4.9 |
Falx | 9 | 5.6 |
Cerebral convexity | 9 | 5.6 |
Sphenoid ridge | 15 | 9.3 |
Olfactory groove | 2 | 1.2 |
Optic sheath | 2 | 1.2 |
Tuberculum sellae | 3 | 1.9 |
Lateral ventricle | 8 | 4.9 |
Third ventricle | 1 | 0.6 |
Cavernous sinus | 29 | 17.9 |
Tentorial | 13 | 8.0 |
Falcotentorial | 1 | 0.6 |
Cerebellopontine angle | 29 | 17.9 |
Petroclival | 28 | 17.3 |
Clivus | 4 | 2.5 |
Cerebellar convexity | 1 | 0.6 |
Total | 162 | 100.0 |
Variables | Range | Median |
Diameter (mm) | 7.8–44.4 | 24.8 |
Volume (cm3) | 0.3–50.4 | 10.4 |
Max. dose (Gy) | 18.6–50.0 | 29.9 |
Marg. dose (Gy) | 10.0–25.0 | 14.8 |
No. of isocenters | 1–21 | 6 |
Illustrative Case
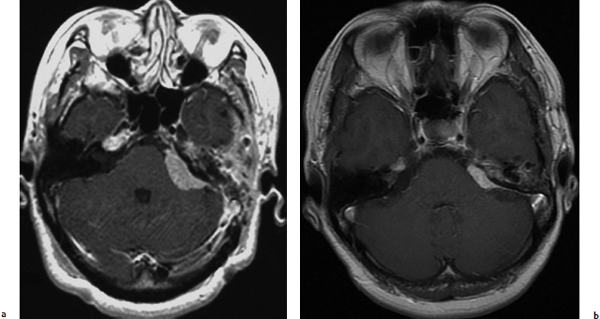
A 50-year-old woman with left facial numbness underwent partial resection of a petrous tumor diagnosed as a WHO grade I meningioma (Fig. 4.7a). GKRS was directed to the residual tumor with maximum and marginal doses of 32 Gy and 16 Gy, respectively. The tumor volume had markedly decreased 15 years after treatment (Fig. 4.7b), and the patient did not develop any neurologic deterioration after radiosurgery.
Long-Term Tumor Control
For patients harboring WHO grade I meningiomas, long-term tumor control is essential. So far, numerous investigators have documented the safety and efficacy of radio- surgery for benign meningiomas.38–47 But there is little information regarding long-term tumor control or late adverse radiation effects such as tumorigenesis more than 20 years after radiosurgery. In other words, it may not be uncommon to develop treatment failure or a secondary neoplasm several decades after treatment. This issue is the reason why radiosurgery for young patients remains controversial. The only way to resolve this issue is to keep following the patients who underwent radiosurgery.
In their large radiosurgical series of 972 patients harboring meningiomas in various locations with a mean follow-up period of 48 months, Kondziolka and associates43 reported that the actuarial 5- and 10-year tumor control rates were 93% and 87%, respectively. They delivered a mean marginal dose of 14 Gy to the tumors with a mean volume of 7.4 cm3. The overall tumor control rates depended on the confirmed histology of the tumor, with 97% in tumors without previous histological confirmation, 93% in WHO grade I tumors, 50% in grade II tumors, and 17% in grade III tumors. The actuarial tumor control rates past 10 years were 91% and 95% in grade I tumors and in those without previous histological confirmation, respectively. In our experience, 19 patients developed in-field tumor progression 14 to 151 months after GKRS. Two of 82 patients (2.4%) who were followed longer than 10 years had in-field tumor progression, indicating that treatment failure past 10 years is uncommon. In another radiosurgical series of 200 patients harboring skull-base meningiomas, Kreil and associates44 showed that the actuarial 5- and 10-year tumor control rates were 98.5% and 97%, respectively. Their median marginal dose was 12 Gy and the median tumor volume was 6.5 cm3. As described in our experience, most commonly, patients with skull-base meningiomas achieve higher tumor control rates than those with supratentorial meningiomas when treated with radiosurgery. Conversely, the complete resection of skull-base meningiomas without any morbidity is definitely more difficult than that of supratentorial meningiomas such as convexity or falx lesions. Thus, a higher long-term tumor control rate with radiosurgery is expected with a lower morbidity rate than surgical resection for the illustrative case of the 39-year-old presented at the beginning of this section.
Fig. 4.7a,b (a) T1-weighted image with gadolinium enhancement showing a well-enhanced petrous meningioma at the time of gamma knife radiosurgery. (b) T1-weighted image with gadolinium enhancement showing that the tumor had decreased markedly 15 years after treatment.