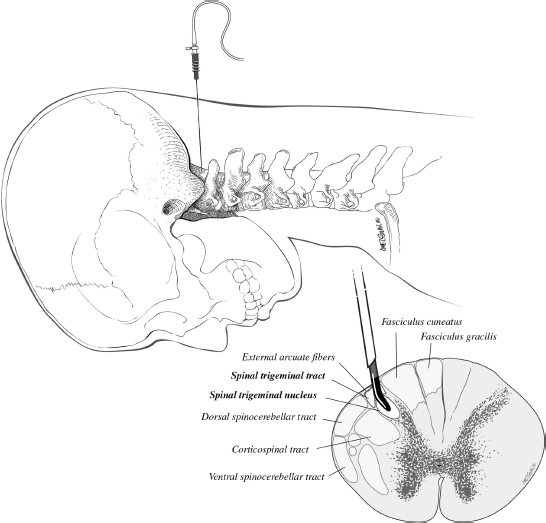
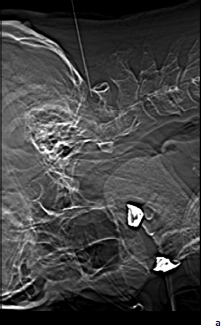
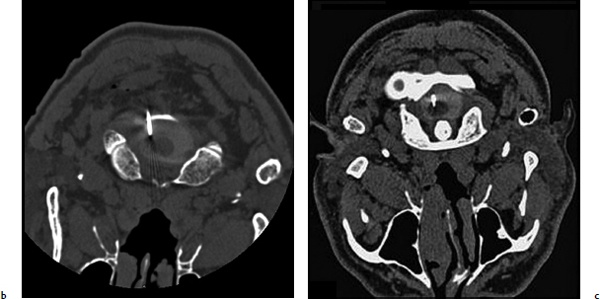
Chapter 20 Case A 50-year-old man underwent resection of a petroclival meningioma. Three months after surgery, he began to have facial pain that was persistent and increasing, associated with dysesthesia, and intractable to all forms of medical treatment. Participants Percutaneous Descending Trigeminal Tractotomy–Nucleotomy for Intractable Facial Pain: Yucel Kanpolat Central Neurostimulation for Intractable Neuropathic Facial Pain: Esmiralda Yeremeyeva, Chima Oluigbo, and Ali Rezai Moderator: Treatment of Intractable Facial Pain: Kim J. Burchiel In the 1996 edition of Controversies in Neurosurgery, three great masters of neurologic surgery—Schvarcz,1 Burchiel,2 and Gybels3—provided some unique recommendations for a patient with neuropathic pain after the removal of a petroclival meningioma. With my 40 years’ experience in medical pain practice, I would emphasize the following points regarding this problematic case: 1. Drugs usually fail to resolve neuropathic pain; nevertheless, I still recommend antidepressants or gabapentin for such patients. 2. Peripheral lesions are usually contraindicated and worsen the neuropathic pain; thus, they are useless. 3. The efficacy of stimulation is unsatisfactory. 4. Tractotomy-nucleotomy is preferable for such patients as a first step. If it is only partially effective, a nucleus caudalis dorsal root entry zone (DREZ) lesion can be created as a final stage. The practice of central lesioning of the trigeminal tract, which contains the fibers of the fifth, seventh, ninth, and tenth nerves of pain areas, was initiated by Sjöqvist4 in 1938 and designated as trigeminal tractotomy. The procedure was done as open surgery. In 1970, Hitchcock5 described a stereotactic percutaneous trigeminal tractotomy, and in 1972, Crue and colleagues6 published their description of radiofrequency trigeminal tractotomy. In view of the lesioning of the oral pole of the nucleus caudalis, the procedure was termed trigeminal nucleotomy by Schvarcz (personal communication, 1993).7,8 Sindou9 performed lesioning of the substantia gelatinosa and named the procedure selective posterior rhizotomy. Nashold and associates10,11 used the procedure with radiofrequency electrodes and named it the DREZ operation. In 1987, Bernard and colleagues12 described the procedure as nucleus caudalis DREZ lesions. I began to use computed tomography (CT) imaging in the practice of destructive pain procedures and CT guidance for lesioning of the trigeminal tract and nucleus.13–17 Over a period of 23 years (1988–2011), I performed 95 trigeminal tractotomynucleotomy procedures in 80 patients and 13 nucleus caudalis DREZ operations in some special pain cases.17 Tractotomy-nucleotomy was used as a special method for understanding the benefit of the nucleus caudalis DREZ operation. In 12 of the 13 patients undergoing the nucleus caudalis DREZ procedure, trigeminal tractotomy-nucleotomy was effective, but not sufficiently so, and I decided to carry out a nucleus caudalis DREZ operation in these patients.17 Fig. 20.1 Schematic drawing of the percutaneous puncture of the occiput-C1 level 6 to 8 mm lateral from the midline, and the target-electrode relationship and main anatomic structures in the percutaneous tractotomy-nucleotomy. The procedure is done with the patient lying face down, and a posterior approach is preferred (Fig. 20.1). The target is located at the occiput-C1 level. The needle or cannula is inserted either freehand or stereotactically 6 to 8 mm from the midline at the occiput-C1 level. The neck is flexed and the target is one third of the lateral part of the ipsilateral upper spinal cord. The depth of the electrode is planned according to the diametral measurements of the spinal cord in this region, 3 to 3.5 mm from the posterior surface of the spinal cord. This method has been described previously.13–17 The first part of the procedure entails localization of the needle electrode system morphologically; after the morphology is determined, the physiological part of the procedure becomes crucial (Fig. 20.2). The lesion side is chosen through stimulation, with the first branch of the trigeminal nerve at the most distant point anteriorly and the seventh, ninth, and tenth nerves at the most distant point posteriorly. Stimulation and impedance measurement are mandatory at this point. Impedance is usually more than 1,000 Ohm and the stimulation parameters are started from the lowest level: 2 to 5 Hz, 0.03 to 1 V. The procedure is painful, and patients sometimes cannot tolerate the high standard-frequency electrical stimulation. The final stage of the procedure is lesioning. The surgeon must remember that lesioning of the nucleus caudalis or descending trigeminal tract is painful. As the patient cannot tolerate high standard lesions, Schvarcz recommended that I elevate the caudal extent of the curved electrode tip in an upward and downward motion. However, I have been unable to use the technique because of my own lack of sufficient experience in this regard. Over the past 23 years, I have performed 95 procedures in 80 patients. Most of them had craniofacial cancer pain, atypical facial pain, and glossopharyngeal, geniculate, and vagal neuralgias. Definitive diagnoses included 17 patients with craniofacial cancer pain, 15 with atypical facial pain, four with geniculate neuralgia, and 17 with glossopharyngeal neuralgia. The rest of the patients had mixed pain and were not given one definitive final diagnosis. In this group, there was only one neuropathic pain problem immediately after the removal of a petroclival neurofibroma from the cerebellopontine angle. The diagnosis was neuropathic pain, and the procedure was not effective. As a last choice, we performed 13 nucleus caudalis DREZ operations. In 12 of them, the tractotomy-nucleotomy was effective. Based on this result, we can state that, if the tractotomy-nucleotomy is effective, there is hope that the nucleus caudalis DREZ lesion will be even more effective. I consider the tractotomy-nucleotomy as my first-line destructive procedure in this neuropathic central pain condition. If the procedure is only partially effective, I can use nucleus caudalis DREZ lesioning for further efficacy. Fig. 20.2a–c The percutaneous approach to the occiput-C1 level in the trigeminal tracto- nucleotomy. (a) Lateral scanogram of the procedure. (b) Final position of the cannula. (c) Final position of the electrode. The presented case represents the most problematic type of patient in neurosurgical practice, and there is no standard algorithm for treatment decisions. Based on my personal experience, I recommend tractotomy-nucleotomy for such patients. If it is only partially effective, as a final procedure I would recommend a nucleus caudalis DREZ lesion. Chronic neuropathic pain has a significant impact on the quality of life of 70 million Americans.18 The economic burden of chronic pain is reflected in health care costs, missed work days, and high unemployment rates. Patients with chronic neuropathic pain experience a substantially lower health-related quality of life than the general pop- ulation.19 Chronic neuropathic pain is mainly characterized by a constant pain often accompanied by amplified responses to both noxious and nonnoxious stimuli. It results from a lesion anywhere in the afferent nociceptive pathways. Associated symptoms may be negative (hypoesthesia, hypoalgesia) or positive (sensitivity to cold or heat, hyperalgesia, paresthesias, allodynia).20 Pain to the face can be referred, but most pain sensation is through the trigeminal nerve, which provides the sensory innervations to the face.21 The causes of intractable neuropathic facial pain are numerous—tumors, the herpes virus (shingles), malignant or benign neoplasms, multiple sclerosis, infections, sarcoidosis, orofacial surgery—or they may be idiopathic. In contrast to trigeminal neuralgia, neuropathic facial pain is continuous, burning, or aching, although there are some fluctuations in the severity. Because the sources of pain can be various or even unknown, it is particularly hard to treat this pain with medication. According to Burchiel’s22 new classification of facial pain, classic trigeminal neuropathic pain (TNP) is a consequence of an accidental injury to the trigeminal nerve, for example, from trauma or surgery. Deafferentation facial pain results from intentional injury to the nerve through ablative procedures, with the goal of treating trigeminal neuralgia or neuropathy. Other types are trigeminal post-herpetic neuralgia in a dermatomal distribution, facial pain from multiple sclerosis, and atypical facial pain, which is secondary to a somatoform pain disorder.22 Various mechanisms may be responsible for the development of chronic neuropathic pain and its accompanying negative and positive symptoms. Wallerian degeneration and its consequent surrounding inflammation upregulate receptors for neurotrophic factors and cytokines, thus increasing the excitability of the primary sensory neurons. This phenomenon creates an exaggerated response to the peripheral stimulus and manifestations of allodynia and mechanical hypersensitivity. Another effect of peripheral nerve injury is the development of new contacts between the sensory neurons and the sympathetic nervous system. This development has been shown in two investigations in which α2-subtype receptors, which are normally present on dorsal root ganglion sensory neurons, were increased in models of peripheral nerve injury.23,24 The connectivity of sensory afferents may also be abnormal because of novel synapse formation in the dorsal horn.25 Opioid medications are generally not effective for treating patients with chronic neuropathic pain, and there have been relatively few new alternatives, such as antiepileptics and antidepressants. Clinical trials show that pharmacotherapy in clinical practice offers only 40 to 50% symptomatic pain relief and no changes in the pathophysiology of the disease.26 Several neuromodulation surgical options, such as central and peripheral stimulation, exist. In this section we focus on two central neurostimulation modalities used to treat neuropathic facial pain: motor cortex stimulation (MCS) and deep brain stimulation (DBS). The general surgical approach in patients with chronic neuropathic pain must be done by a multidisciplinary team that includes a pain specialist, who assesses the patient first and ultimately exhausts all the medical options; a neuropsychologist, who determines if there is significant cognitive impairment and active psychiatric issues; and a neurosurgeon. A social support system is imperative in caring for these patients postoperatively as well. In 1991, Tsubokawa and colleagues27 attempted to stimulate the sensory and motor cortex as components of the somatosensory pathway and noted efficacy from stimulation of the motor cortex. In their cat model, stimulation of the motor cortex completely and persistently inhibited the burst hyperactivity of thalamic neurons. They theorized that thalamic pain syndromes can be addressed through chronic stimulation of the motor cortex. They proceeded to stimulate 12 patients with medically refractory central deafferentation pain and achieved a 67% rate of a lasting decrease in pain.28 In 1993, Meyerson and colleagues29 treated their patients with motor cortex stimulation (MCS) and noted that all patients with TNP had significant lessening of their pain. In the past 15 years, there has been rising interest in this modality of pain treatment, as demonstrated by Fontaine and associates30 in their review of 244 MCS studies of chronic neuropathic pain (1991–2006). Despite this growing interest, the exact mechanism of action is still unknown. Positron emission tomography studies of patients undergoing MCS implicate the thalamus as the key structure mediating functional MCS effects.31,32 One hypothesis derived from functional studies is that descending axons activated by MCS from the motor and pre-motor cortices in turn activate thalamic nuclei, which cycle with other pain-related structures receiving afferent pain perception, such as the medial thalamus, anterior cingulate cortex, and upper brain stem.31,32 Another hypothesis points to an increase in the secretion of endogenous opioids triggered by chronic MCS.33 Motor cortex stimulation is mainly used to treat central thalamic pain, TNP,30 and, less so, peripheral neuropathic pain. Other indications reported in the literature include phantom limb pain, brachial plexus avulsion, spinal cord injury, post-herpetic neuralgia, and peripheral nerve lesions, including nerve root or nerve trunk pain related to previously excised tumors.34–39 Recording a detailed history of the patient is imperative to confirm the diagnosis, and a neuropsychological evaluation must be done to determine the presence of ongoing uncontrolled depression, anxiety disorders, substance abuse, or other major psychological issues, which must be addressed and stabilized before surgery. The patient should understand that a trial period of several days with the electrodes is required. A positive response must be at least a 50% reduction in pain; if the pain is not reduced during the trial, the electrodes are removed without further consideration of MCS. The general sequence of events is preoperative localization of the motor cortex, intraoperative electrophysiological mapping of the face area of the motor cortex, implantation of the MCS electrodes followed by an externalized MCS trial, and then internalization of the system after a successful trial. The face area of the motor cortex can be localized through a fusion of preoperative images with the neuro-navigation system, which enables anatomic localization of the motor cortex. Functional magnetic resonance imaging (MRI) and transcranial magnetic stimulation may also be used (Fig. 20.3). Electrodes may be placed through bur holes or a small craniotomy. A craniotomy may allow a more accurate intraoperative electrophysiological evaluation. The procedure is done with the patient either under general endotracheal anesthesia or, in some centers, awake under monitored anesthesia care. Paralytic agents are not used, as they block the electromyographic responses used for electrical cortical mapping. The patient’s head is fixed with a three-pin headholder to eliminate motion and to allow the use of the frameless navigation system. The outline of the precentral gyrus is projected onto the scalp with preoperative imaging fused with the neuronavigation system, and the incision is centered over this region. We prefer a 4-cm craniotomy, which is large enough to allow the epidural placement of a 4 × 4 electrode grid. The 16-electrode testing grid is then placed in the epidural space (Fig. 20.4).
Treatment of Intractable Facial Pain
Percutaneous Descending Trigeminal Tractotomy–Nucleotomy for Intractable Facial Pain
Central Neurostimulation for Intractable Neuropathic Facial Pain
Surgical Intervention
Motor Cortex Stimulation
Background
Surgical Technique
< div class='tao-gold-member'>
Treatment of Intractable Facial Pain
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree