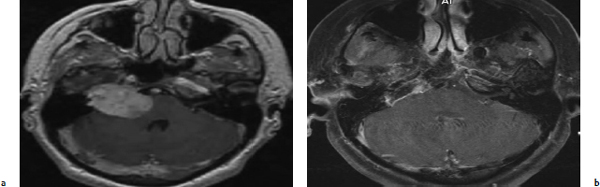
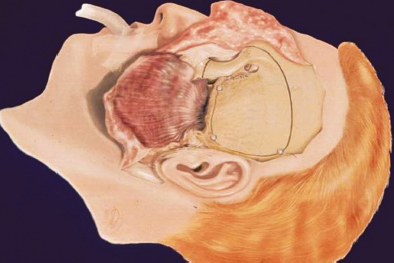
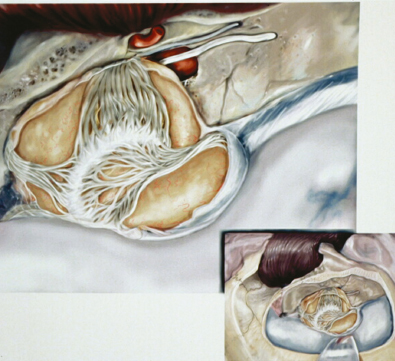
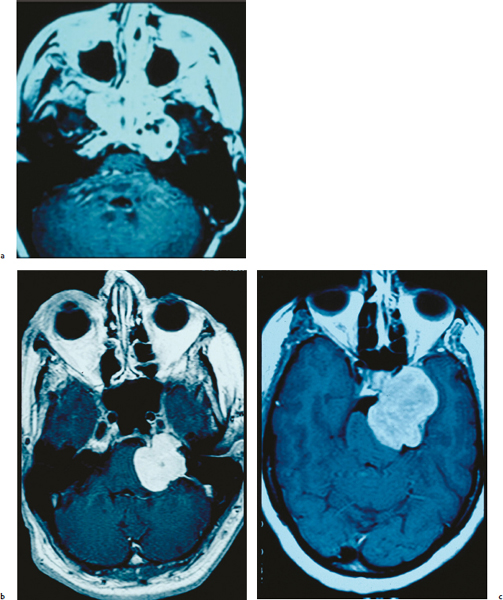
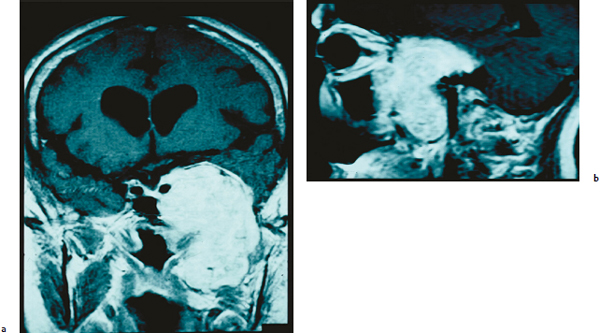
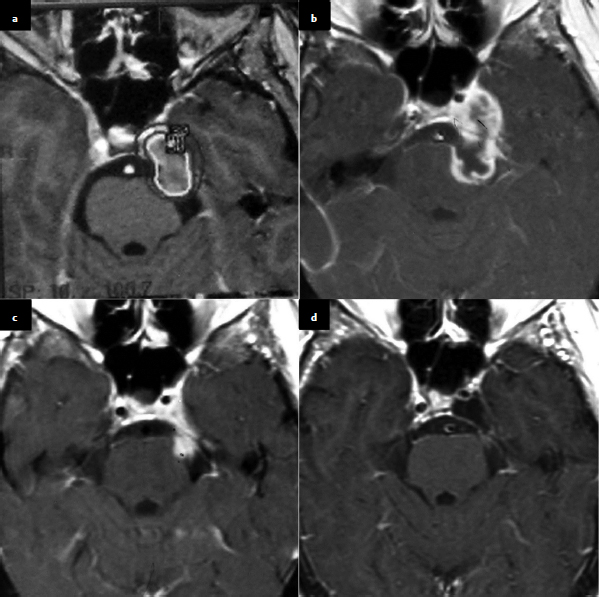
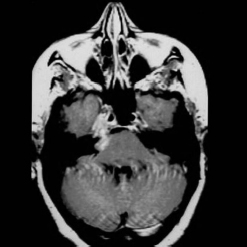
Chapter 6 Case A 40-year-old patient comes for medical treatment for facial pain and numbness with mild hypoalgesia and hypoesthesia in V2 and V3. Participants Total Removal of Trigeminal Schwannomas: Cristian Gragnaniello and Ossama Al-Mefty Stereotactic Radiosurgery for Trigeminal Schwannomas: Douglas Kondziolka, Hideyuki Kano, and L. Dade Lunsford Moderator: Management of Trigeminal Schwannoma: Microsurgical Removal vs. Radiosurgery: Takeshi Kawase The imaging study shows a dumbbell-shaped right-sided skull-base lesion with a large component in the middle fossa that extends into the posterior fossa. It is highly enhancing and further extends into the cavernous sinus anteriorly and toward the brainstem in its posterior component. The imaging study shows a certain degree of bony erosion of the petrous bone. This patient presented with facial pain and numbness, hypoalgesia, and hypoesthesia in V2 and V3. The clinical presentation and the radiological findings are consistent with the diagnosis of a trigeminal schwannoma rather than a neurofibroma; neurofibromas most likely involve the cavernous sinus but do not typically extend posteriorly further than Meckel’s cave.1 Trigeminal schwannomas are rare, benign lesions accounting for 0.07 to 0.5% of all intracranial tumors and 0.8 to 10% of all schwannomas. They arise from the Schwann cells of the nerve sheath and therefore may be located anywhere along the entire course of the nerve. As reported in the literature, 20% of trigeminal schwannomas emerge from the cisternal segment, 50% from Meckel’s cave, and 5% from the distal intracranial segment. On magnetic resonance imaging (MRI), schwannomas are usually iso- or hypointense on T1-weighted images and hyperintense on T2-weighted images, and when contrast is administered they show either homogeneous or rim enhancement.1 The surgical approach for such a tumor is tailored to each patient, and proper selection is important to achieve total removal, improve preoperative neurologic deficits, and alleviate postoperative deficits (Fig. 6.1). Trigeminal schwannomas often involve the cavernous sinus space and extend to the posterior fossa. Therefore, skull-base approaches have shown many advantages for treatment, such as shortening the distance to the lesion, eliminating retraction of the brain, and facilitating the possibility of working below the temporal lobe, while protecting the draining veins below the intact dura. In particular, the extradural zygomatic middle fossa approach allows the removal of large dumbbell-shaped trigeminal schwannomas and offers a multiplicity of working angles to reach the tumor in its infratemporal, intramaxillary, intrasphenoidal, or intraorbital extension and in the cavernous sinus. Furthermore, the portion extending into the posterior fossa and compressing the brainstem can also be reached through the expanded Meckel’s cave (Fig. 6.2).2,3 The patient is placed in the supine position with the ipsilateral shoulder elevated and the head rotated to the contralateral side, with the zygoma almost parallel to the floor. The skin incision is carried 1 cm anterior to the tragus in a curvilinear fashion, behind the hairline, to the superior temporal line. The superficial temporal artery and the frontotemporal branches of the facial nerve are preserved. The skin flap is dissected anteriorly and the thick areolar tissue is left with the pericranial flap. At this stage, to preserve the facial nerve, an incision is made 1 cm posterior and parallel to its course along the zygomatic arch, keeping the nerve protected between the superficial and deep temporal fascia and the fat pad. The two fascias and the fat pad are then reflected anteriorly with the skin flap. A subperiosteal dissection of the temporal muscle, incised posteriorly to the superficial temporal artery, is carried from the root of the zygoma forward to protect the middle temporal artery. With the B1 attachment, the zygoma is detached with oblique anterior cuts through the malar eminence and through the root, posteriorly. The temporal muscle is detached at the level of the superior temporal line and reflected down with the zygoma to access the middle fossa. A bur hole is made at the level of the temporal fossa floor, a temporal craniotomy is designed, and the flap is elevated (Fig. 6.3). The surgeon should localize important landmarks in the middle fossa to prevent injury to the neurovascular structures. The dura of the middle fossa is elevated from the temporal fossa floor to expose and divide the middle meningeal artery at the foramen spinosum with a posterior-to-anterior and lateral-to-medial dissection to prevent injury of the greater superficial petrosal nerve, which could result in facial palsy. This nerve exits the facial hiatus and runs in a groove on the floor of the middle fossa in an anteromedial direction, passing under Meckel’s cave to reach the foramen lacerum, where it joins the deep petrosal nerve to form the vidian nerve. The greater superficial petrosal nerve is also an important landmark for locating the petrous segment of the internal carotid artery and gaining proximal control over the cavernous internal carotid artery. Fig. 6.1a,b Axial contrast-enhanced magnetic resonance imaging (MRI) studies depicting a trigeminal schwannoma that expands into Meckel’s cave and extends in a dumbbell shape into both the middle and posterior fossae. (a) Preoperative image. (b) Postoperative image showing total removal. The foramen rotundum and the maxillary division of the trigeminal nerve are identified lateral and posterior to the superior orbital fissure. The foramen ovale is located 1 cm posterior and lateral to the rotundum and transmits the mandibular division of the nerve. The outer dural layer over the trigeminal division is elevated to expose the lower cavernous sinus and peeled medially to expose the tumor in that location (Fig. 6.4). The lesion can be reached between the divisions of the nerve or behind the ganglion. The tumor is carefully debulked with suction because of its soft consistency, and it is followed posteriorly through the expanded Meckel’s cave. Once debulked, it is carefully dissected from the roots, ganglion, and divisions to save the fascicles that are not involved with the tumor. Fig. 6.3 Artist’s illustration of the middle fossa zygomatic approach, including the skin incision, preserving the facial branches, sectioning of the zygoma, and the bone flap. There is no need to drill the petrous apex or section the tentorium because the enlarged mouth of Meckel’s cave offers a natural pathway between the middle and posterior fossae to follow the lesion into the posterior fossa. If needed, the mouth can be enlarged with an upward incision toward the superior petrosal sinus, which might require coagulation and sectioning of its most anterior part. In the posterior fossa, the lesion can be safely dissected from the brainstem, rootlets, and basilar artery if an intraarachnoidal plane of dissection is maintained (Fig. 6.5). Fragments of the tumor may be present in the infratemporal and pterygoid fossae; these can be excised after the floor of the middle fossa is removed between the foramen ovale and the rotundum. Fragments along the superior orbital fissure can be extirpated to remove the middle fossa floor between the foramen rotundum and the superior orbital fissure. If the middle fossa floor is drilled, it can be reconstructed with a vascularized temporal muscle flap to prevent cerebrospinal fluid leaks.3 Trigeminal schwannomas are benign and slow-growing lesions; however, their location has been the cause of morbidity in the past. These tumors can arise from any segment of the nerve (root, ganglion, or any one of the three divisions) and extend into more than one compartment, resulting in a more challenging treatment. This is particularly true in the case described here. The first classification of trigeminal schwannomas proposed by Jefferson in 1959 was modified by Day and Fukushima4 and others to include different features of the tumor, such as the degree of extension into the multiple fossae and the degree of bony erosion of the petrous apex4,5 (Table 6.1) (Figs. 6.6, 6.7). In earlier cases, these lesions were removed through conventional approaches but with high morbidity and recurrence reaching 70% and 65%, respectively.6,7 Some authors have noted the difficulty in removing dumbbell-shaped schwannomas via the subtemporal and retrosigmoid approaches.8 The introduction of skull-base approaches and, more recently, extradural approaches has proved helpful in tackling the intradural portions of the lesion. The additional extradural approach used by Dolenc9 and Day and Fukushima4 enhanced the results of previous series. We found the zygomatic middle fossa approach and the zygomatic osteotomy, respectively, advantageous in achieving extradural total removal and minimizing retraction on the temporal lobe while providing multiple working angles. The zygomatic middle fossa approach exposes the tumor in every location, even in the posterior fossa. We maintain, however, that a different approach is needed for the very caudal extension of the tumor below the facial and acoustic nerves.3 Fig. 6.5 Artist’s illustration of the surgeon’s view after the posterior fossa extension of the tumor has been reached and removed through Meckel’s cave, exposing the basilar artery and the brainstem. (From Al-Mefty O, Ayoubi S, Gaber E. Trigeminal schwannomas: removal of dumbbell-shaped tumors through the expanded Meckel cave and outcomes of cranial nerve function. J Neurosurg 2002;96:453-463. Reprinted by permission.) Because this lesion is benign, it does not invade neural or vascular structures that are only displaced. Dissection from the fibers of the trigeminal nerve is possible with microsurgical techniques to preserve and improve nerve function (75% improvement of facial pain and 80% improvement of trigeminal motor function). The introduction of skull-base approaches to the treatment of trigeminal schwannomas offers the possibility of total removal with very good outcomes and low morbidity. During skull-base procedures, intraoperative neurophysiological monitoring offers the surgeon additional safety in removing lesions that involve and displace cranial nerves. More specifically, this type of monitoring allows the surgeon to locate the involved cranial nerves, which confirms nerve function during surgery and forecasts the surgical outcome. Table 6.1 Jefferson’s Classification of Trigeminal Schwannomas
Management of Trigeminal Schwannoma: Microsurgical Removal vs. Radiosurgery
Total Removal of Trigeminal Schwannomas
Case Presentation
Surgical Approach
Discussion
Type | Description |
A | Tumors located mainly in the middle fossa that arise from the gasserian ganglion |
B | Tumors located predominantly in the posterior fossa that arise from the root of the trigeminal nerve |
C | Tumors with significant components in both middle and posterior fossae |
Dumbbell-shaped tumors |
In a previous report, the senior author (O.A.) analyzed the microsurgical total resection of 25 trigeminal schwannomas, of which only 24% involved the middle fossa alone and 76% involved both the middle and posterior fossae. All tumors involved the cavernous sinus. After surgery, all pre-operative deficits of the cranial nerves (rather than just the trigeminal nerve) and all brainstem and cerebellar symptoms were alleviated. There was a remarkable improvement in 75% of patients who presented with facial pain, 80% of those suffering from motor trigeminal weakness, and 44% of those with facial numbness. Only 13% of the lesions recurred and 12% of patients had worsening or new deficits in trigeminal function. The mean age of the patients at the time of treatment was 44 years.3
Pamir and colleagues10 reported on a series of 18 patients in which 50% of the lesions were Jefferson type C trigeminal schwannomas and only two patients had a tumor smaller than 2 cm. They reported one recurrence and one subtotal removal, and 16 patients were free of tumor at 134 months. Zhou and associates11 described a series of 57 cases of dumbbell-shaped Jefferson type C trigeminal schwannomas (50% more than 40 mm in diameter and 26% more than 50 mm). They reported a total removal rate of 87% and only one recurrence at a mean follow-up of 10 years. The neurologic function of cranial nerves improved in 93% of patients with facial numbness, 83% with deteriorating trigeminal motor function, 80% with palsy of the sixth and seventh nerves, and 75% with palsy of the ninth and tenth nerves and cerebellar or brainstem signs. They treated 38 patients with the gamma knife with a 65-month follow-up, and reported that 83% of the tumors decreased in size, 5.7% remained stable, and 8.6% increased with worsening trigeminal function in 25% of patients. They also reported four patients in whom surgical removal was incomplete but the residual tumor did not grow during long-term follow-up. In only one case (25%), it grew after 5 years, requiring further treatment.
In 2006, Moffat and colleagues12 reported a small series of eight patients surgically treated. This report is important because of the characteristics of the lesions, which were more similar to those routinely radiated (50% Jefferson type A). The outcome was excellent: 100% of facial pain and 62.5% of facial numbness resolved. Sarma and associates13 reported on 26 trigeminal schwannomas with a gross total resection in 100% of patients and the preservation of cranial nerve function, which reflects our experience. It is important to emphasize that, in 100% of patients, they were able to dissect the tumor from the trigeminal nerve, preserving its function in all patients with a medium-sized tumor (2.5 cm or less). Yoshida and Kawase8 reported a comprehensive series of trigeminal schwannomas involving multiple fossae. The lesions operated on through skull-base approaches were totally removed in 15 of 18 patients. Two of the recurrences, representing 11% of the series, had been treated first at other institutions. Only four patients had tumors of 3.5 cm or less whereas the others had a mean diameter of 4.9 cm (range 3.5 to 8 cm). In the small series of five trigeminal schwannomas reported by Mariniello and associates,14 all the tumors were located in the cavernous sinus. The authors observed an improvement of facial pain in all patients with no recurrences or complications.
Recently, different series of stereotactic radiosurgery (SRS) have shown good control of tumor growth in patients with small trigeminal schwannomas (less than 3 cm in diameter) with a short follow-up. It is very difficult to compare the results of surgery and radiosurgery in the treatment of trigeminal schwannomas for multiple reasons, including the different size of treated lesions, the different times of onset of complications, and the short follow-up for radiosurgery. Regardless, it is the comparison between the two modalities that is the focus of the controversy. Hasegawa and colleagues15 treated 37 patients affected with trigeminal schwannomas using the gamma knife. The mean dose delivered to the tumor was 27.9 Gy and the marginal dose was 14.2 Gy. Only 22% of the tumors were type C (dumbbell shaped) and only 16% of the patients had compression of the brainstem with deviation of the fourth ventricle. At 54 months of follow-up, there was good tumor growth control in 84% of patients whereas 14% suffered from an enlargement of the lesion or uncontrollable facial pain with radiation-induced edema that required surgical extirpation. There was no difference in the outcome of patients who underwent gamma knife surgery as a first treatment or after previous surgery. The functional outcome of the cranial nerves improved in 40% of the patients, with worsening or the onset of new symptoms in 14%.
Phi and associates16 treated 22 patients and reported at 2-year follow-up. Eleven tumors were dumbbell shaped and 82% of patients underwent radiosurgery as a first treatment. The outcome showed persistence in trigeminal pain in 27% of patients, facial hypoesthesia in 63%, motor trigeminal weakness in 15%, and sixth nerve palsy in 50%. Facial dysesthesia was permanent in 13.6% of patients and 4.5% experienced worsening of preexisting trigeminal pain. New deficits were observed in 27% of patients. Overall, there was good tumor growth control, with only 4.5% of patients having uncontrolled lesions. The most interesting data in the series relates to the presence of tumor in the cavernous sinus, with the development of new deficits in 42.8% of patients. Pan and associates17 reported on a series of 56 patients treated with the gamma knife, with results that overlap those of other reports for the same technique. The data from patients with uncontrolled tumors show an increase in recurrence in those affected by tumors with a diameter greater than 30 mm compared with those affected by tumors of 30 mm in diameter or less by a 3:1 ratio. Huang and colleagues18 examined a series of 16 patients whose tumors were well controlled at 44-month follow-up and found no worsening of preexisting symptoms and improvement of 31. In a series of 23 patients treated for trigeminal schwannomas, Nettel and coauthors19 reported tumor growth in 9% of patients at follow-up, 9% with a new onset of symptoms, and 4.3% with peritumoral changes.
Radiation-induced changes in neural tissue are well known and often have an insidious onset, especially in the long term. Trigeminal schwannomas mostly occur in the third and fourth decades of life in patients with a long life expectancy, but have also been reported in children and young adults.15–17,19 Radiation-induced injuries to small brain vessels are known to manifest shortly after treatment. Radiation vasculopathy of larger vessels, however, is not well recognized, and there are few reports that suggest reconsidering its incidence even with small doses of radiation, as in gamma knife surgery, especially when treating the cavernous sinus and brainstem for benign lesions. We focused on the possibility of dissecting the tumor from the fascicles of the trigeminal nerve using microsurgical techniques. Morita and colleagues20 have suggested that 19 Gy is a safe dosage to be delivered to the trigeminal nerve. When the nerve is affected by the tumor, it is difficult on a radiological image to discern the tumor from the normal healthy nerve while delivering, in most cases, a mean dose of 27 Gy to the tumor.
As we have noted previously, the use of the gamma knife after failed surgery is as safe and effective as its use in patients who were never surgically treated. But this statement is not valid when we analyze data from patients undergoing surgery after the failure of treatment with the gamma knife. In these patients, the tumors adhere tightly to vital structures such as nerves, vessels, and the brainstem, making total removal very difficult and with greater risk. Gwak and associates5 reported that, in cases of subtotal removal of a trigeminal schwannoma, the isolated residual tumor was stable after 5 years in 75% of patients, suggesting that, in cases of subtotal removal, it is worthwhile to wait and observe the residual until it grows. An important limit in the discussion of surgery versus radiosurgery series lies in the fact that most patients included in the surgery series are not suitable for radiosurgery because their lesions are too big to be irradiated. Because trigeminal schwannomas are benign, slow-growing lesions that mostly present in the third and fourth decades of life, we believe they must be treated surgically by experienced surgeons who persistently aim for total removal. Patients with a progressive tumor who are not surgical candidates, or who have residual or a recurrent tumor that cannot be removed, can be referred for radiosurgery, as current reports indicate that this modality induces good control of tumor growth and acceptable cranial nerve outcomes, but at a relatively short follow-up.
Stereotactic Radiosurgery for Trigeminal Schwannomas
Case Presentation
The presented case is that of a 40-year-old with a history of facial pain and numbness, and the neurologic examination noted mild hypoesthesia and hypoalgesia in the second and third trigeminal divisions. The axial contrast-enhanced MRI shows a mass in the right cavernous sinus extending along the course of the trigeminal nerve. It has a dumbbell shape that is typical of a trigeminal schwannoma.
The management of this patient includes both diagnosis and therapy, and the differential diagnosis for a lesion in this location could include a schwannoma, meningioma, lymphoma, metastasis, hemangioma, or nonneoplastic process such as granulomatous inflammation. The latter would be unusual in this case because of the extent of lesion outside the cavernous sinus. A metastasis would be less likely in a patient at this age and without a cancer history. The presentation and shape of the tumor are most consistent with a trigeminal schwannoma. We would therefore make that diagnosis without histology sampling and would manage the patient as such.
Therapeutic options include observation with serial imaging studies, microsurgical resection (complete or partial), SRS, fractionated radiotherapy, or combined approaches. Because this patient is symptomatic, we favor treatment over observation, and because the tumor is fairly small in volume, the patient is an ideal candidate for radiosurgery. In our experience, gamma knife radiosurgery is associated with a high rate of tumor response and regression and possible improvement in symptoms. This could be achieved without exposing the patient to the risks of craniotomy, or to a higher chance of impaired neurologic function or vascular injury.
Background
Trigeminal schwannomas are slow-growing, benign nerve sheath tumors that are uncommon compared with vestibular schwannomas.21–25 They account for less than 1% of all intracranial tumors and 0.8 to 8% of all intracranial schwannomas.7,21–27 Despite continued improvements in cranial-base surgical techniques, including endonasal surgery, a tumor’s adherence to adjacent critical neurovascular structures makes complete removal difficult.7,8,22–24 When such removal is attempted, new postoperative neurologic deficits are common.7,13,18,23,27 The rate of new or worsened trigeminal function after tumor removal varies from 13 to 86%.7,8,22,23,28
There is increasing literature about the value of SRS for patients with trigeminal schwannomas.4,29–34 In a recent review, we retrospectively assessed tumor control, clinical response, the risk of adverse radiation effects, and variables that affect treatment outcomes.26
Clinical Experience with Gamma Knife Radiosurgery
Over a 16-year interval before 2005, 33 patients with trigeminal schwannomas underwent SRS at the University of Pittsburgh with the gamma knife manufactured by Elekta Instruments (Norcross, GA) (Table 6.2). There were 17 males and 16 females with a median age of 49.5 years (range 15.1–82.5 years). Eleven patients (33%) had prior surgical resection and one patient was diagnosed through stereo-tactic biopsy. Twenty-two patients (67%) underwent SRS as primary management. As in the case presented, in our series the diagnosis was based on a combination of clinical symptoms or signs and confirmatory neuroimaging findings. All tumors extended along the course of the trigeminal nerve, had diffuse contrast enhancement identified by MRI, and had no dural tail. Six patients underwent SRS at the time of tumor recurrence identified by imaging. Tumor progression after initial management was defined as an increase in tumor volume delineated on MRI. The median duration between last surgical removal and progression was 22.1 months (range 9.4–76.5 months).
The classification of each lesion was based on the location of the tumor, and the tumors were classified into one of three types: root (tumor predominantly located in the posterior fossa); ganglion (tumor predominantly located in the middle fossa); and dumbbell (tumor involving both the middle and posterior fossa),35 as in the case presented.
In our patients, the median tumor volume was 5.0 cm3 (range 0.5–18.0 cm3), similar to that of the presented case. A median of six isocenters (range 1–13) was used for dose planning. The median prescription dose delivered to the tumor margin was 15 Gy (range 12–22 Gy), and the prescription isodose was 50% in all cases. The maximum dose varied from 24 to 40 Gy (median 30 Gy). All patients had a minimum follow-up of 6 months (range 7.2–147.9 months), and 24 patients had a follow-up of 24 months or more. The mean follow-up time was 6 years.
Table 6.2 Characteristics of Patients (n = 33) in the University of Pittsburgh Series
Median age (range) | 49.5 (15.1–82.5) |
Male | 17 |
Female | 16 |
Type: Root | 6 |
Ganglion | 17 |
Dumbbell | 10 |
Solid | 31 |
Cystic | 2 |
Prior surgery | 11 |
Mean/median target volume (cm3) (range) | 5.0/4.2 (0.5–18.0) |
Mean/median margin dose (Gy) (range) | 15.0/15.0 (12–20) |
Mean/median maximum dose (Gy) (range) | 29.9/30.0 (24–40) |