Chapter 18 Case A 65-year-old patient in otherwise good health has symptomatic 80% carotid occlusion. Participants Stenting for Carotid Stenosis: Rabih G. Tawk, Adnan H. Siddiqui, Elad I. Levy, and L. Nelson Hopkins Advocating Carotid Endarterectomy: Markus Bookland and Christopher M. Loftus Moderators: Managing Symptomatic Carotid Stenosis: Endarterectomy vs. Stenting: Ning Lin, A. John Popp, and Kai U. Frerichs This case is an excellent illustration of the difficulties associated with the decision-making process for patients with carotid artery disease. For this otherwise healthy patient, four essential approaches are possible: no treatment, medical treatment, endovascular intervention with carotid artery stenting (CAS), and open surgery with carotid endarterectomy (CEA). At our center, we prefer to define the degree of stenosis on the basis of a diagnostic cerebral angiogram, the study that continues to represent the gold standard for the evaluation of carotid disease. The diagnostic angiogram elucidates several factors that are essential to the decision-making process for selecting the optimal treatment for a given patient. In addition to the severity of stenosis, several essential elements guide our decision before recommending a treatment modality in general and in particular for this patient who is in good health. In this section, we review key studies comparing CAS to CEA and outline the advantages and current and practical indications for CAS. Our perspective is that of neurosurgeons trained in both open and endovascular surgery, and we define carotid lesions primarily on the basis of findings on the diagnostic cerebral angiogram. Because this patient has a symptomatic lesion, treatment with either CAS or CEA would be superior to medical treatment. In the presented case, we would evaluate the lesion’s characteristics and the patient’s candidacy for both CAS and CEA before making a final recommendation for or against CAS. Carotid artery disease is implicated in approximately 25% of ischemic stroke cases.1 With advancements in endovascular technology and techniques and increasing experience and expertise among surgeons, the role of CAS in stroke prevention has continued to evolve. Many treatment centers now consider CAS to be a first-line, less invasive therapeutic alternative to CEA for patients at high risk for surgical complications. Subsequent to the publication of the results of the North American Symptomatic Carotid Endarterectomy Trial (NASCET)2 and European Carotid Surgery Trial (ECST),3 in which the benefits of CEA over the best available medical therapy were demonstrated, the Carotid Revascularization Endarterectomy versus Stent Trial (CREST)4 and the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial5 affirmed that CAS is not inferior to CEA for certain subgroups of patients. Although the precise role of CAS in primary and secondary stroke prevention has not been defined, data from multiple reports have demonstrated the efficacy of CAS for treating patients with carotid artery stenosis.6–9 Consequently, the safety and the widespread use of CAS have created a dilemma regarding which method of carotid revascularization to use in which population, particularly in patients considered at high risk for CEA, as defined by NASCET.10 Because the NASCET analysis included a highly selected group of patients and excluded those at high risk for surgery (Table 18.1), several newer trials documented the efficacy of CAS in both high-risk and standard-risk patients and compared these results with those from well-established reports on CEA.4,9–13 The SAPPHIRE5,13 and the Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS)11 trials suggested that the stenting procedure is not inferior in short- and long-term follow-ups of mixed cohorts of symptomatic and asymptomatic patients. The 30-day outcomes of the Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) study,14 the Stent-supported Percutaneous Angioplasty of the Carotid artery versus Endarterectomy (SPACE) study,2,9,15 and the International Carotid Stenting Study (ICSS)16 have been analyzed extensively. Although EVA-3S14 and SPACE12 failed to reach the prespecified margin for the noninferiority of CAS compared with CEA for 30-day outcomes, longer term follow-up of these studies showed low and similar rates of ipsilateral stroke at 2 and 4 years of follow-up, respectively, in both treatment groups. Recently, CREST demonstrated that the risk of the composite primary outcome of stroke (myocardial infarction) or death during the 30-day periprocedural period or ipsilateral stroke within 4 years after randomization did not differ significantly between CAS and CEA among standard-risk patients with symptomatic or asymptomatic carotid stenosis.4 The incidence of stroke during the periprocedural period was lower in the CEA group than in the CAS group, and the incidence of periprocedural myocardial infarction and cranial nerve palsy was lower in the CAS group. The countervailing effects in the periprocedural event rates resulted in similar rates of primary outcomes in the two groups. After the periprocedural period, the ipsilateral stroke rates in CREST were 2.0% with CAS and 2.4% with CEA (similar to the rates in the SPACE and EVA-3S trials), suggesting excellent durability for up to 4 years. The carotid stenosis population represents a heterogeneous mixture of individuals with specific underlying medical comorbidities and specific lesion characteristics. According to our experience, no single treatment can serve all patients as one group.17 Both CEA and CAS are effective treatment options, and we believe that the treating physician should be able to match every patient to the technique that is safer, while realizing the highlights and the pitfalls of the existing data on each. Although the results from major trials that compared CAS to CEA (CREST, SPACE, EVA-3S) are similar to a certain degree with respect to specific parameters, CAS is particularly attractive in certain subgroups of patients, especially those who were excluded from the NASCET2,10 because of associated medical “high risk” and poor surgical candidacy. The high-risk features for CEA that were adopted as criteria for including participants in CAS registries and trials are listed in Table 18.2. Therefore, selection of either CAS or CEA is not only about a better or a more durable technique but also requires attention to the patient’s age, medical comorbidities, lesion characteristics, and proximal vascular anatomy (with respect to endovascular access). Further, as stent design, delivery systems, and cerebral embolic protection devices continue to be refined, the long-term outcomes for CAS continue to improve and the procedural risks continue to decrease. Table 18.1 Criteria of Exclusion from the North American Symptomatic Carotid Endarterectomy Trial (NASCET)2
Management of Symptomatic Carotid Stenosis
Stenting for Carotid Stenosis
Background
Selection of Treatment Modality: CAS vs. CEA
The following criteria were used to exclude patients from the study: 1. Age 80 years or older 2. Mentally compromised or unwilling to give consent 3. Both carotid arteries and their intracranial branches could not be visualized 4. An intracranial lesion was more severe than the surgically accessible lesion 5. Failure of the kidney, liver, or lung, or a cancer was judged likely to cause death within 5 years? 6. Cerebral infarction on either side that deprived the patient of all useful function in the affected territory 7. Symptoms that could be attributed to nonatherosclerotic disease(e.g., fibromuscular dysplasia, aneurysm, or tumor) 8. Cardiac valvular or rhythm disorder likely to be associated with cardioembolic symptoms 9. Previous ipsilateral carotid endarterectomy The following criteria were used to determine that patients were temporarily ineligible*: 1. Uncontrolled hypertension, diabetes mellitus, or unstable angina pectoris 2. Myocardial infarction within the previous 6 months 3. Signs of progressive neurologic dysfunction 4. Contralateral carotid endarterectomy within the previous 4 months 5. Major surgical procedure within the previous 30 days |
Advantages of CAS
In the absence of detailed guidelines that specify the use of CEA or CAS, patients should be offered an approach that is both individualized and lesion-specific. We consider CEA and CAS as complementary methods for specific patient populations and lesions, and the assignment of a technique is not dichotomized for one technique versus the other. Indeed, patients at high risk for CEA are often low risk for CAS and vice versa. This objective evaluation allows us to select the optimal treatment modality for each patient, as most would be better served with a specific modality. From a technical standpoint, CAS is a minimally invasive procedure and does not require neck dissection to expose the carotid artery, which is challenging especially in patients after radiation therapy or surgery of the neck area. Exposure is also very challenging in patients with a short neck and a lengthy stenotic lesion. In addition, CAS avoids prolonged brain hypoperfusion because no occlusion of the carotid artery is required. In CEA, the internal carotid artery (ICA) is occluded during resection of the lesion; thus, we favor CAS especially in patients with contralateral occlusion. Further, CEA-associated complications—mainly, cranial nerve palsies—are avoided. Moreover, some patients request the less invasive endovascular CAS procedure; thus, patient preference drives the approach selected as well.
Carotid artery stenting is particularly advantageous in several subgroups of patients, especially those with a variety of medical comorbidities. Such patients are deemed at high risk and therefore considered poor surgical candidates, yet could be easily treated with CAS. Another subgroup that would benefit substantially from CAS is represented by patients harboring very high lesions located toward the skull base. The retraction and dissection required to expose high lesions is substantial, and such lesions are associated with a high risk of cranial nerve injuries (in 7.6 to 27% of patients).10,18 Patients who have undergone previous neck surgery have scar tissue that obscures the neurovascular structures and places them at an increased risk of injury.19 Restenosis, which occurs in 1.5 to 49% of endarterectomy patients,20,21 constitutes another technical challenge for CEA. With increasing patient longevity, medical comorbidities are increasingly encountered and carotid disease recurs with greater frequency. CAS circumvents the need to operate through scarring and permits the treatment of lesions located at the proximal and distal ends of the endarterectomy sites, which are difficult to access surgically. Because the complication rate associated with a repeated CEA can be as high as 10.9%, CAS is likely safer than a second CEA in cases of recurrent stenoses.19,22–24 Another setting in which CAS is particularly beneficial is for patients in whom lesions are caused by diseases other than atherosclerosis, such as radiation-induced stenosis and fibromuscular dysplasia. Radiation-induced stenoses tend to be longer than atherosclerotic lesions, often extending from the origin of the ICA to the skull base, and are usually composed of atheromatous debris and sclerotic tissue, which clearly complicates CEA.25–27 Such patients have altered anatomic planes and a poor capacity to heal and, therefore, might be better served with CAS.28,29 Further, patients who develop stenosis after radiation are also more likely to have undergone previous neck surgery, another factor that complicates CEA.
Table 18.2 High-Risk Features for Carotid Endarterectomy
1. A history of myocardial infarction within 30 days 2. Unstable angina with electrocardiographic changes 3. Angina with two-vessel coronary artery disease 4. New York Heart Association class 3–4 congestive heart failure 5. An ejection fraction < 30% 6. Chronic obstructive pulmonary disease with < 30% of predicted forced expiratory volume in 1 second (FEV1) 7. Heart surgery required within 30 days 8. Vascular surgery required within 30 days 9. Age > 75 years 10. High cervical or intrathoracic stenosis 11. A previous ipsilateral carotid endarterectomy 12. A history of radical neck dissection or neck irradiation 13. Bilateral carotid artery stenosis, contralateral occlusion 14. A previous contralateral carotid endarterectomy with cranial nerve palsy 15. Immobility of the cervical spine 16. A crescendo of or recent transient ischemic attacks 17. Tandem stenosis 18. Intracranial hypoperfusion 19. Tracheostomy |
Carotid artery stenting also represents a superior treatment option in patients with symptomatic thrombus, in which the stent can plaster the thrombus against the vascular wall and prevent it from distal migration. Likewise, CEA is difficult in the setting of carotid dissection because of the typically distal location and long extension of these lesions compared with atherosclerotic stenosis. Open surgical treatment of carotid dissections is associated with a high complication rate and normally consists of proximal vessel ligation, direct repair, embolectomy, or bypass, because CEA is generally not recommended.30,31 For these lesions, CAS represents a superior option32–40 and is now the treatment of choice for symptomatic dissections not responding to anticoagulants.32,41,42 Stenting can reapproximate an intimal flap against the wall and maintain patency of the lumen. Endovascular techniques also permit possible treatment of associated pseudoaneurysms. In the absence of guidelines for the management of ICA dissections, we recommend stenting for cases in which the patient experiences ischemic symptoms despite medical therapy, for cases involving radiographic progression of the lesion, and for cases in which the stenosis exceeds 80%.
Another scenario in which we favor CAS is for cases of contralateral ICA occlusion. Compared with patients with unilateral disease, CEA is associated with a higher rate of complications in patients with contralateral occlusion, and, according to the NASCET investigators, the 30-day risk of stroke and death approaches 14.3%.2 This risk is likely attributable to the time needed for ICA clamping during CEA (NASCET median, 32 minutes) in patients with poor intracranial collateral circulation. We have found CAS particularly advantageous in this setting because CAS avoids prolonged ICA occlusion, which is typically limited to 5 to 15 seconds to inflate the angioplasty balloon.43 Further, we typically perform CAS with the patient under conscious sedation with continuous neurologic evaluation. In these settings, clinically significant complications (such as embolization of plaque that can lead to stroke) may be managed or treated immediately through the existing proximal access route.
To date, CAS outcomes likely have been hindered by our limited knowledge of atherosclerotic lesions and inappropriate selection of patients. Currently, the need for CEA or CAS is dictated solely by the degree of lumen obstruction, and many centers use only Doppler imaging, which is operator-dependent, to assess the severity of the stenosis. Recently, it has been shown that the histology, morphology, and composition of carotid plaques play a major role in and seem to influence the outcomes of CAS to a greater degree than those of CEA.44 Although angiography is poor in detecting the composition of atherosclerotic lesions, virtual histology, a new technology incorporated into intravascular ultrasound equipment, allows a histological characterization of plaques by creating a reproducible analysis of the radiofrequency and amplitude data of the ultrasound waves that cross different tissues. By characterizing the morphology, extension, and histology of the plaque, this technique provides important information to confirm the percentage of stenosis and judge its embolic potential, tailor the procedure, guide the choice of stent, and check stent apposition and complete coverage of vulnerable plaques. We have had a favorable experience with intravascular ultrasound during CAS,45 and virtual histology intravascular ultrasound has the potential to further optimize patient and lesion selection criteria for CAS to improve procedure-related outcomes.44
Conclusion
In our opinion, carotid revascularization with CAS or CEA, performed by highly qualified surgeons with dual training, is safe and effective. When assessing patients with ICA stenosis, we consider the relative risks associated with CEA and CAS and their applicability to specific patient populations and lesion types. CAS continues to be particularly attractive for several groups of patients, especially those at increased risk for undergoing CEA (e.g., patients who were excluded from the NASCET). Our practice of open and endovascular surgery performed by neurosurgeons with dual training represents an attempt to increase safety and completeness in the management of neurovascular disorders, and perhaps this practice model will bridge the gap between open and endovascular subspecialties and pave the way for treatment of cerebrovascular disorders in the future.
Advocating Carotid Endarterectomy
As a disease entity, carotid artery stenosis rarely presents without serious and compelling comorbidities. Such comorbidities must be factored into any treatment strategy for carotid artery stenosis and to evaluate the merit of medical treatment alone, endovascular treatment (namely CAS), and surgical reconstruction (namely CEA) in search of the optimal therapy for these patients. Illustrating the benefits of CEA, we present a 64-year-old patient with severe bilateral carotid artery stenosis.
This patient presented initially with a sudden onset of right-sided central facial palsy and upper extremity paresis. She had a history of peripheral vascular disease and severe congestive heart failure. A standard stroke workup uncovered a left internal capsule infarct and bilateral carotid artery stenoses (Fig. 18.1). The patient had not previously sought treatment for her constellation of medical problems and was therefore referred initially for medical optimization. Her primary medical providers recommended she begin aspirin therapy and undergo placement of a defibrillator, and they consulted with our department for treatment of her stenosis. A left CEA was recommended, and the patient agreed to the procedure after a full discussion of the risks and benefits.
Procedure
The preparation of the patient for CEA began with general endotracheal intubation, the placement of an arterial line, and concurrent somatosensory evoked potential (SSEP) monitoring and electroencephalograph (EEG) for redundant electrophysiological monitoring during surgery. This preparation is consistent with our experimental protocol at Temple University School of Medicine.
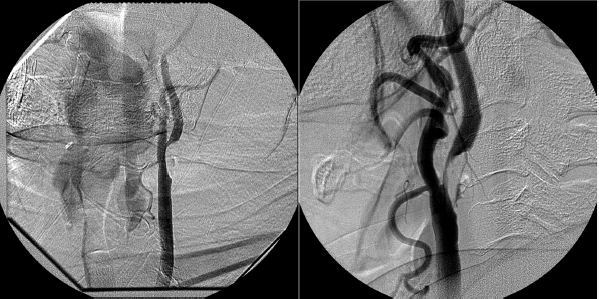
Fig. 18.1 Angiograms of the left and right common carotid arteries, respectively, showing more than 70% stenosis of the bifurcations.
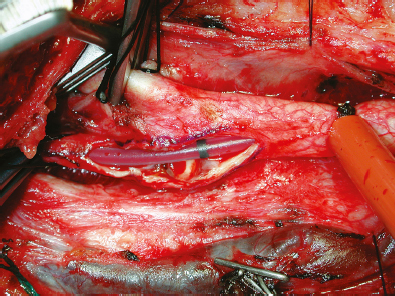
Fig. 18.2 The Loftus shunt can be seen bridging the arteriotomy from the common carotid artery to the internal carotid artery. The black stripe indicates the middle of the tubing and can be used to detect migration of the catheter.
Next, in preparation for cross-clamping the carotid vessels, we asked the EEG and SSEP technicians to obtain baseline readings. The ICA was occluded first, followed by the CCA, and finally the external carotid artery was closed. The arteriotomy was begun in the CCA with a No. 11 scalpel blade and followed with Potts angled scissors. The blue-marked arteriotomy line was followed from the CCA up to the bifurcation, and then up the ICA until normal vessel lumen was encountered.
Some small left-sided EEG/SSEP changes were noted upon clamping of the ICA; for this reason, a Loftus carotid endarterectomy shunt (Integra LifeSciences, Plainsboro, NJ) was carefully passed into the CCA and secured by pulling up on the silk tie surrounding the artery (Fig. 18.2). A small Scanlan–Loftus custom ICA pinch clamp was used to secure the shunt after its passage into the distal ICA. With the plaque exposed and carotid blood flow controlled, a Freer plaque dissector was then used to gently dissect the plaque away from the arterial wall.
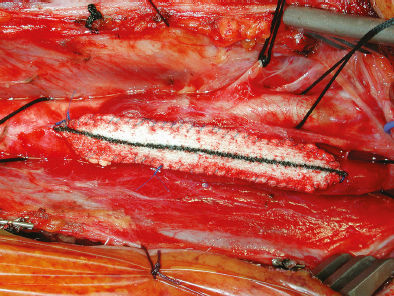
Once the vessel wall was clean and smooth, we repaired the arteriotomy defect with a Hemashield patch graft, as is our routine. A running, nonlocking 6–0 Prolene suture (BV-1 needle) stitch was used to close the fitted patch to the arteriotomy walls (Fig. 18.3). To remove the Loftus shunt, a small opening was temporarily left on the lateral CCA wall to retrieve the catheter.
With the arteriotomy fully repaired and the shunt removed, the de-clamping sequence began. A heparinized saline syringe with a blunt tip was inserted into a small lateral wall opening while the two stitches abutting the syringe were held tight. The vessel was filled with heparinized saline, while a surgeon’s knot was thrown and laid down against the syringe. The final release of the arterial clamps then proceeded in the following sequence: first the external carotid artery, then the CCA, and, after a 10- second pause, the ICA.
Next, the retractors were removed and wound hemostasis was ensured by direct inspection. Carotid patency was once again confirmed with the handheld Doppler. A layered closure followed, including the carotid sheath, platysma, and skin. Subsequent to closure, the patient was awakened and extubated without complications; she was neurologically and hemodynamically stable.
A more extensive discussion of our recommended detailed surgical technique can be found in our carotid texts.46–48
CEA vs. CAS
Surgical intervention is the most highly effective treatment option for patients with carotid artery stenosis.2 There is, however, a subset of high-risk patients with carotid artery stenosis and significant comorbidities, such as advanced age, poor cardiac function, and limited pulmonary reserve, that justifies consideration of alternative therapies. For this reason, some have advocated stenting (namely CAS) as a suitable alternative to CEA when dealing with complicated circumstances and frail patients.
Fig. 18.3 The finished arteriotomy patch repair. The CCA, ICA, and external carotid artery have all been isolated with 0 silk ties. Toward the top of the image, the protected vagus nerve can be seen, and the hypoglossal nerve lies within a rubber loop just along the lateral edge of the figure.
The case presented above is an example of just such a patient. Her cardiac condition was complex, and the patient had been recommended for cardiac catheterization and defibrillator placement at some point in the perioperative period. Additionally, the patient had a significant oxygen requirement at baseline and suffered near constant dyspnea. Although we agree that these comorbidities warrant consideration of a stent, CAS, somewhat surprisingly, has simply not proved to be as effective or safe as surgical carotid reconstruction.
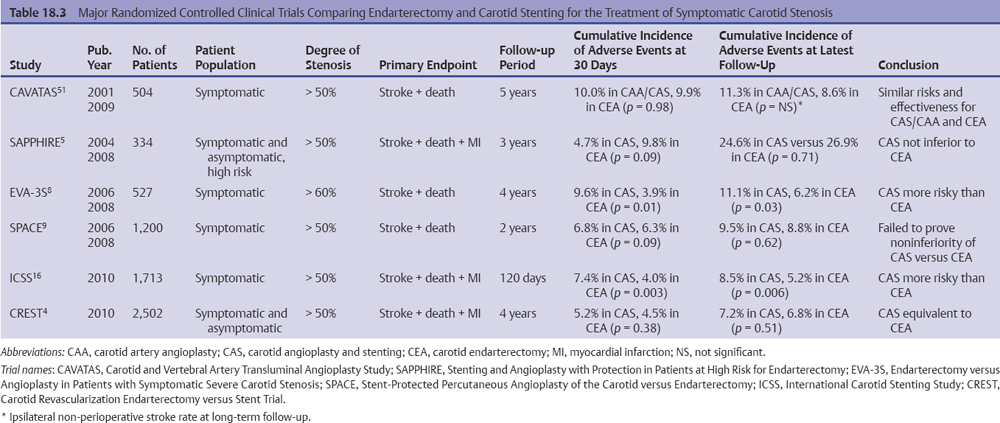
We have reviewed 11 reports of randomized controlled trials comparing CEA and CAS.5,8,9,11,13,49–54 The results have varied, but they have universally failed to validate CAS as a superior treatment for carotid artery stenosis. In fact, several have found CAS to have a much higher risk of sub-acute cardiovascular events than does carotid surgery. In 1998, the Leicester trial, though limited by a small sample size, found a 45% increase in periprocedural cardiovascular events among CAS patients compared with CEA patients. In 2001, the WALLSTENT multicenter randomized controlled trials found an alarming rate of ipsilateral strokes and deaths among CAS patients (CAS 12.1% vs. CEA 3.6%).52,54 Several randomized controlled trials have since attempted to reverse the stigma of 30-day and 1-year infarcts from carotid stenting, but these have largely failed. The SAPPHIRE trial, released in 2004, stands as the lone exception. We note, however, that the trial included an enormous number of patients, nearly all receiving stents, who were treated outside of the trial, which always introduces the specter of a selection bias against surgery. In addition, the SAPPHIRE results that claim equipoise, unfortunately, have not been reproducible in subsequent, larger randomized controlled trials.5,13
In the absence of equipoise, then, for low-risk patients, CAS has been most strongly advocated for treating patients with carotid artery stenosis who have significant comorbidities. Elderly patients are often considered to be a high-risk group for whom CAS might be attractive. However, several prospective and retrospective studies have compared CEA and CAS in the elderly and found that age does not significantly alter the results. A review of 53 patients older than 70 years at the University of Iowa Hospital found no differences in CEA outcomes in these patients when compared with historic, younger controls.55 Similarly, and alarmingly, a meta-analysis of eight CAS study groups and 33 CEA study groups that selected for patients over 80 years of age identified increased rates of postprocedural stroke among CAS patients, similar to those rates noted in past randomized controlled trials. No other differences were seen in periprocedural or long-term mortality and myocardial events in these studies.56
Some groups have recommended CAS rather than CEA for patients with unfavorable cervical anatomy, the so-called hostile neck. Patients with previous neck surgeries, high carotid bifurcations, irradiated cervical fields, or a nonextendable neck may be considered more efficaciously approached through an endovascular route. A case-controlled trial in 2009 addressed this question by prospectively pairing and following 154 CEA patients. In the final analysis, no difference in periprocedural ischemic events or mortality was noted.57 Although a direct comparison between CAS and CEA patients with difficult anatomy has yet to be done, these initial data would give credence to the thought that the hostile neck has little bearing on CEA outcomes when a skilled surgeon performs the procedure.
Carotid artery stenting (with distal protective devices) has been an innovative new technique for the treatment of carotid stenosis, and no doubt has its place in the management of this disease. But the evidence to date is unequivocal and strongly favors surgical repair. No high-risk group yet stratified has shown improved results with CAS when compared with CEA, and in broader-based randomized controlled trials there has been consistent and reproducible evidence for a lower 30-day stroke and death rate for patients randomized to CEA. The results of the CREST trial, comparing endovascular and surgical treatment for non–high-risk carotid stenosis patients, are eagerly awaited as this chapter was being written. In the absence of novel data to support CAS, however, current evidence-based medicine continues to confirm the superiority of CEA over CAS for treating patients with carotid stenosis.
Moderators
Managing Symptomatic Carotid Stenosis: Endarterectomy vs. Stenting
From the outset, it should be stated that the senior author of each of the above two sections (L.N.H. and C.M.L.) not only are thought leaders in their respective fields but also have extensive experience and have been involved with the evolution of the management paradigm for symptomatic carotid stenosis over the past three decades. Although we find little fault with what has been stated by either group of authors, we believe that the issues being explored may be much more nuanced than the “either/or” scenarios proposed by the two sections. These nuances form the substance of our discussion.
In essence, Bookland and Loftus advocate endarterectomy as superior to carotid stenting with lower periprocedural stroke and death rates, and they have highlighted the importance of the technical proficiency of the operating surgeon. Tawk and associates emphasize the benefit of carotid stenting in certain subsets of patients with symptomatic carotid stenosis, while taking an evolutionary view of the field by asserting the complementary nature of endarterectomy and stenting. In this section, we provide a review of the currently available literature on the treatment of symptomatic carotid stenosis and discuss the relative advantages and limitations of these two approaches.
Early Clinical Trials
Atherosclerotic stenosis of the carotid artery is an important cause of cerebral ischemia and is estimated to be responsible for up to 20% of all ischemic strokes.51,58,59 The management of carotid bifurcation atherosclerosis as a source of stroke first received detailed conceptual and pathoanatomic attention from C. Miller Fisher in the early 1950s.60 Thereafter, reports of successful surgical treatment of carotid bifurcation atherosclerosis followed.61 Between 1950 and 1991, opinions about the utility of CEA fluctuated considerably due to conflicting findings regarding the risks, benefits, and proper indications for the procedure.62–65 A series of randomized controlled clinical trials in the 1990s performed in North America2,66 and Europe67 unequivocally demonstrated that CEA combined with medical therapy is superior to medical therapy alone for stroke prevention in certain symptomatic patients with high-grade carotid stenosis. As a result of these trials, for nearly two decades CEA was established firmly as superior to the best medical treatment of the era for certain patients with symptomatic carotid stenosis.
Initially, the technique for endovascular treatment of carotid stenosis was balloon angioplasty, which was first introduced in 1980s.68–70 Early, nonrandomized case series showed the potential benefits of an endovascular approach, including no surgical incision, a need for only local anesthesia, and a shorter length of stay, but there remained a substantial concern about the safety of the procedure.52,68,71,72 One of the first randomized controlled trials, the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS),51 compared the results of carotid balloon angioplasty with or without stenting to endarterectomy in patients with symptomatic carotid stenosis (Table 18.3). This study showed that the 30-day stroke or death rate was similar in both groups (approximately 10%) and that minor complications such as cranial nerve palsies and wound complications occurred more frequently in the CEA group. Although the study highlighted the benefits of a minimally invasive approach, the periprocedural stroke/death rate was high in both groups (10.0% for stenting vs. 9.9% for endarterectomy), and stenting was used only in 26% of the patients. Long-term results (median follow-up of 5 years) assessed by determining the rate of non-perioperative stroke (greater than 30 days after the procedure) did not differ between the CAS and CEA groups (1.4% vs. 1.1% per year, respectively),73 but the study itself was not designed to detect a significant difference.
< div class='tao-gold-member'>