Chapter 199 Management of Median Nerve Compression
Introduction to Median Nerve Compression
Due to their superficial locations, close relationship to neighboring muscles and tendons, and often long and meandering courses, peripheral nerves are frequently subject to physical forces that may result, if applied chronically, in functional impairment or neuropathy. When a nerve is exposed to repetitive stretch, compression, or friction, progressive internal injury may occur and sometimes debilitating symptoms of weakness, paresthesias, or pain will be manifest in the distribution of the affected nerve. The most common occurrence of peripheral nerve compression is that of the median nerve at the wrist, also known as carpal tunnel syndrome (CTS).1 In this chapter, we will characterize the phenomenon of median nerve entrapment not only at the wrist, but also in the arm, at the elbow, and in the proximal forearm. We will describe the clinical features, anatomic findings, and surgical management relevant to compression at each site, including current evidence-based recommendations and technological innovations, such as endoscopy. We hope to provide the reader with both a general understanding of peripheral nerve entrapment and an up-to-date approach to the diagnosis, evaluation, and treatment of median nerve compression syndromes.
Etiology of Nerve Entrapment
The mechanisms underlying peripheral nerve injury due to entrapment are multifactorial and variable in nature, as an individual nerve may be more or less likely to suffer the effects of stretch, pressure, or friction based on its proximity to the skin surface, its axonal density, and the composition of the tissue in which it travels. A relatively superficial nerve that angles sharply around bony prominences, squeezes through packed fibrous tunnels, or is exposed to repetitive intervals of lengthening and shortening, as with flexion and extension across appendicular joints, is at greater risk for developing a compressive neuropathy. Forces may be applied to the nerve in large amounts for short periods of time (acute mechanical compression) or in small amounts on a more chronic basis. Several animal and human studies have demonstrated that early disruption of myelinated fibers, development of extra-axonal edema, and subsequent changes in axoplasmic flow may ultimately lead to a reduction in membrane excitability, axonolysis, and Wallerian degeneration.2–8 Systemic diseases such as diabetes mellitus, advancing patient age, and local vascular insufficiency or venous stasis may also contribute to neuronal ischemia and reduced regenerative capacity that, in combination, can exacerbate neural fibrosis and cause progressive neuropathy.9,10
Clinical Presentation, Diagnosis, and Treatment of Nerve Entrapment
Typically, a patient presenting with symptomatic peripheral nerve compression will relay a history or chief complaint of reduced sensation, paresthesias, pain, and/or weakness in the distribution of the affected nerve that may be worsening over time. Physical examination directed at elucidating alterations in the function of the nerve believed to be involved may reveal a reduction in two-point discrimination and perception of changes in pressure, vibration, or temperature, or decreased power and atrophy of target muscle groups. Specific tests include maneuvers that exacerbate the extent of entrapment, such as manual compression or tapping of an irritable nerve near the skin to cause radiating dysesthesias in the relevant dermatome (Tinel’s sign). In addition to a detailed motor, sensory, and reflex assessment, electromyography (EMG) and nerve conduction velocity (NCV) studies are useful, minimally invasive tools that will potentially confirm or refute suspected deficits discovered on the exam. Findings in entrapment can include prolonged motor or sensory distal latency and delayed or even absent motor or sensory conduction velocity, and these electrical tests will help rule out other diagnoses like motor neuron disease or cervical radiculopathy. Radiographic examinations such as x-ray, computed tomography (CT), and ultrasound (US) may be employed to identify bony abnormalities, soft tissue lesions, or tumors.11 Magnetic resonance imaging (MRI) provides greater detail for peripheral nerve masses such as schwannomas or neurofibromas, while magnetic resonance neurography (MRN) is a newer and more controversial technology that aims to image nerves directly; MRN has shown promise in the evaluation of proximal peripheral nerve compressive lesions (piriformis and thoracic outlet syndromes [TOS]) that may otherwise be difficult to diagnose, but the technique is not yet universally accepted or available.12
Once a diagnosis of peripheral nerve entrapment has been made, treatment is directed at conservative measures that will attempt to reduce the amplitude or frequency of compressive forces on the affected nerve. These steps may include modification of occupational or recreational activities that elicit symptoms, use of splints or immobilizing braces to minimize movements that exacerbate the problem, and prescription of anti-inflammatory medications or corticosteroid injections to reduce nerve edema and irritation. Many patients will experience significant improvement or even resolution of their symptoms with these simple interventions. The peripheral nerve surgeon will be asked to intervene when a patient has failed conservative management but ideally has not yet suffered irreversible loss of neural function. The specific operative approach will be determined by the site of entrapment and requires a familiarity with regional anatomy. Most cases can be completed without risk of major morbidity or further nerve injury, but collaboration with additional specialists, such as vascular or orthopedic surgeons, may be indicated for more complex operations. The basic objective centers on freeing or releasing the compressed nerve from its point of entrapment without traumatizing that nerve or exposing it to prolonged ischemia. Usually, patients recover readily and the desired outcome is achieved. We will discuss multiple aspects of these surgical techniques in greater detail in the paragraphs that follow.
Entrapment of the Median Nerve at the Wrist: Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS), which results from entrapment of the median nerve under the transverse carpal ligament in the wrist, is the most frequently encountered compressive neuropathy. Accounting for more than 200,000 operative procedures annually in the United States alone, this disorder is estimated to occur in 125 of 100,000 people.1,13–15 Affecting women more commonly than men (ratio of more than 2 to 1), CTS often arises in middle age (40–60 years) and among workers in occupations that involve repetitive use of the hands or wrists.16–18 Although it may result from a congenital narrowing of the carpal tunnel compounded by activities that further reduce the size of the carpal tunnel or exacerbate compression of the median nerve, CTS is associated with a myriad of systemic and local causes. Metabolic etiologies include diabetes mellitus, hyper- or hypo-thyroidism, acromegaly, and pregnancy, with CTS impacting upward of 60% of pregnant women; fortunately, appropriate treatment of the underlying disorders and delivery of the baby will usually achieve resolution of the CTS symptoms.19–21 Traumatic injuries like a Colles fracture that heals improperly, mass lesions such as a ganglion cyst, local infections, edema, and anatomic variants may all increase the volume of the contents of the carpal tunnel and entrap the median nerve.
Surgical Anatomy
The carpal tunnel is a canal with its lateral walls and floor created by the carpal bones and their ligamentous attachments, while its roof is formed by the flexor retinaculum or transverse carpal ligament (TCL). The contents of the carpal tunnel include the nine long digital flexor tendons, synovium, a vascular bundle, and the distal median nerve. The TCL itself is a wide fibrous band that begins 1 cm proximal to the distal wrist crease and ends 3 cm distal to the crease, attaching radially to the trapezium and scaphoid tuberosity and ulnarly to the pisiform and hook of the hamate. The median nerve gives off a palmar cutaneous branch as the main trunk emerges from underneath the FDS tendon approximately 3 to 4 cm before the distal wrist crease. This sensory branch usually exits from the radial or anterolateral surface of the median nerve and then travels lateral to the palmaris longus tendon and superficial to the TCL to innervate the proximal thenar eminence.22,23 The median nerve most commonly passes through the tunnel without branching, and then gives off the recurrent motor or thenar branch as it exits the tunnel. Usually arising from the radial side of the median nerve just distal to the TCL, the recurrent motor branch will then curve back to innervate the abductor pollicis brevis (APB) and opponens pollicis muscles within the thenar mass. The peripheral nerve surgeon must recognize, however, the multiple anatomic variations that exist in the branching pattern of the recurrent motor nerve in order to protect it during exposure and division of the TCL. In 30% of cases, the recurrent branch arises from the ulnar side of the nerve and crosses over the median nerve toward its target; in 20% of cases, it pierces the flexor retinaculum and may be subject to entrapment itself; it may also rarely be duplicated.24,25 The most distal branches of the median nerve proceed to the first two lumbricals and subserve sensation for the palmar surfaces of the thumb and lateral two fingers as well as the radial portion of the ring finger.
Clinical Features
The most common presentation of CTS includes complaints of painful paresthesias or burning, numbness, and tingling in the lateral half of the hand and the radial three fingers. Patients may complain of a deep ache or “pins and needles,” and they importantly note an absence of symptoms in the little finger. Occasionally, pain will radiate proximally into the forearm or even into the arm above the elbow, and patients frequently note an exacerbation with activities involving the hand or wrist. Usually the dysesthesias are worst in the thumb and index finger and classically increase at night after extensive hand use during the day; a patient may often be awakened from sleep with a numb hand that he or she must shake or run under water to obtain relief. Sunderland hypothesized that this phenomenon arises when nocturnal venous stasis activates the symptoms and resolves when shaking improves venous return.26 In more advanced neuropathies, the patient may observe decreased strength in the thenar muscles with concordant wasting.
On physical examination, it is common to appreciate a decreased ability to discriminate light touch or painful stimuli in the radial half of the hand and the first, second, third, and radial half of the fourth digits. Gentle percussion over the median nerve just below the distal wrist crease may elicit tingling dysesthesias in the median nerve distribution (Tinel’s sign). Although supposedly “pathognomonic” for CTS, this test has a sensitivity of only 20% to 70% and specificity of 70% to 83% in several studies.27–29 Forced flexion of the wrist for approximately 60 seconds (Phalen’s sign) may also reproduce the patient’s symptoms in CTS; a positive Phalen wrist flexion test appears to be more specific for the diagnosis than does a positive Tinel’s sign.30,31 We find that simple manual compression over the TCL for 30 to 60 seconds is perhaps the most useful provocative maneuver in CTS. The physician may note a variable amount of thenar atrophy involving the opponens pollicis, APB, or flexor pollicis brevis (FPB) muscles, which will be most obvious when comparing the affected hand to the normal contralateral hand. These muscles can be evaluated by asking the patient to bring the thumb medially to touch the little finger (opponens pollicis) and to abduct the thumb from the plane of the palm against resistance (abductor pollicis).
Electrophysiologic studies are very helpful objective measures in confirming the diagnosis of CTS; research has shown abnormal electrodiagnostic studies in over 90% of patients with clinical CTS.32 The palmar sensory-evoked response test provides the earliest and most sensitive indicator of CTS, that of a prolonged distal sensory latency with decreased amplitude or absence of the evoked response. This study is conducted by stimulating sensory fibers in the palm or fingers and recording over the wrist; as with any test, careful performance and interpretation by an expert in electrophysiology is an absolute requirement, as the results may be affected by numerous patient characteristics such as age, weight, and skin temperature. In more advanced cases, distal motor latency may become prolonged (latency greater than 4 milliseconds across the carpal tunnel is diagnostic) and EMG can show the loss of motor units and the presence of denervation potentials with fibrillations and positive sharp waves in the thenar muscles. EMG will also help differentiate CTS from more proximal median neuropathies, C6–C7 radiculopathies or brachial plexus lesions. The aforementioned radiographic examinations may be necessary if there is any suggestion of a neoplastic or non-neoplastic mass lesion or if the electrodiagnostic studies do not corroborate the diagnosis.
Management
Once the diagnosis of CTS has been confirmed, treatment typically involves conservative measures such as behavioral modification, splinting and anti-inflammatory medications. This is particularly true when the etiology is temporary (pregnancy) or part of a systemic disorder (acromegaly, hypothyroidism, etc.) for which appropriate therapies may be implemented. Occupational or recreational activities that exacerbate the symptoms should be avoided or reduced, a wrist splint that maintains the wrist in slight extension or neutral position may be worn, and corticosteroids can be injected into the carpal tunnel, all of which may be effective strategies to decrease or eliminate symptoms. Some experts caution against steroid injections, however, given their temporary benefit and moderate risk of intraneural injection with consequent injury.33–38
Although some patients with clinical CTS will achieve adequate resolution of symptoms with conservative therapy alone, many will not. Indications for surgical decompression include continued or disabling symptoms in the setting of confirmatory electrodiagnostic studies, evidence of muscle weakness or atrophy, and increased two-point discrimination sensory thresholds. A 2008 Cochrane review compared the efficacy of surgical treatment of CTS with non-surgical treatment and found four randomized controlled trials involving 317 patients with CTS; the conclusion of this meta-analysis was that surgical treatment of CTS relieves symptoms significantly better than does splinting, although it was unclear whether this applies to patients with only mild symptoms.39 A recent randomized, multicenter study compared surgical intervention with conservative management in patients with CTS but no denervation, and surgical treatment produced better outcomes at 12 months follow-up.40 In general, most peripheral nerve surgeons believe operative release of the TCL is a safe and reliable treatment for CTS that is most effective when offered early in the disease course prior to extensive axonal damage and loss. Surgical timing is controversial, but a recent retrospective study of 425 patients with CTS demonstrated that the patients who underwent surgery less than 3 years from the time of initial diagnosis were more than twice as likely to have symptom resolution than patients who underwent surgery more than 3 years after diagnosis.32
Conventional (Open) Carpal Tunnel Release
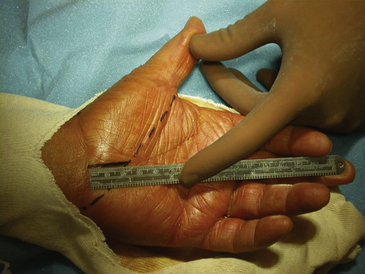
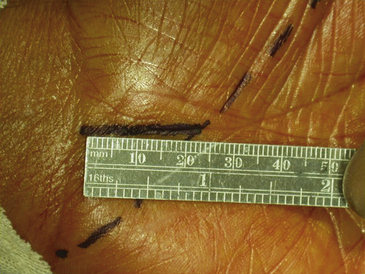
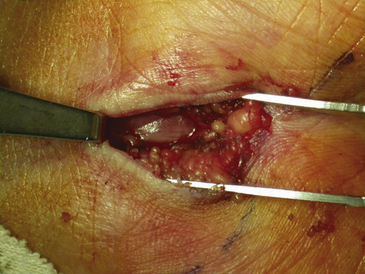
Surgical decompression of the carpal tunnel via transection of the flexor retinaculum was first described by Sir James Learmonth in 1933, and multiple series since have reported patient satisfaction rates of 70% to 96% with the procedure.41 The goal of surgery is to successfully divide the TCL and decompress the median nerve while preserving its recurrent motor and palmar cutaneous branches. The traditional open carpal tunnel release (CTR) is performed using loupe magnification with the patient under locally injected anesthesia (1% lidocaine without epinephrine) with or without mild sedation in an outpatient operating room setting. With the patient positioned supine, the affected arm is abducted and the forearm is supinated on a hand table or an arm board without the use of a tourniquet (to avoid unnecessary ischemia and allow for complete hemostatic control at the conclusion of the case). Following a sterile prep and drape, the incision begins at the distal wrist crease and extends 3 to 4 cm distally in a curvilinear fashion parallel with and approximately 2 mm ulnar to the midpalmar crease (or long axis of the ring finger). The incision ends at or near the level of the distal border of the thumb and may be extended proximally, if necessary, for about 1 cm to create an S-shaped incision. Care must be taken to avoid sectioning branches of the palmar cutaneous nerve during the proximal segment of the skin incision. With experience, the entire incision may be minimized to anywhere from 1.5 to 3 cm in length and still allow for complete division of the TCL (Figs. 199-1 and 199-2).
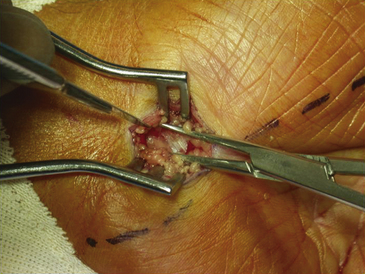
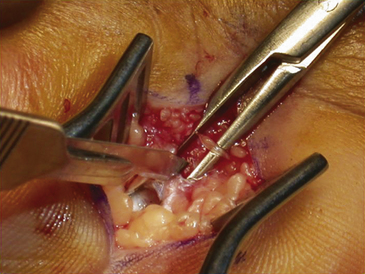
The dissection is then carried down through the subcutaneous fat and the palmar aponeurosis, exposing the TCL, which is aided by the use of small, self-retaining Weitlaner retractors (Fig. 199-3). A no. 15 scalpel is commonly used to start the opening of the firm ligament at its midpoint, revealing, after passing through multiple layers, the underlying median nerve (Fig. 199-4). Once the nerve is exposed, it may be gently dissected away with a No. 4 Penfield blunt dissector or mosquito forceps, and the remainder of the flexor retinaculum may be cut with fine, sharp scissors (iris or tenotomy) or the no. 15 blade; use of the scissors or any other instruments that must be passed beneath the ligament prior to decompression must be done with caution to avoid further compromise of the canal. Additionally, attention must be paid to the fact that the ulnar nerve lies superficial to the TCL and that forceful retraction with the Weitlaner can inadvertently cause compression of the ulnar nerve in Guyon’s canal and result in distal ulnar nerve palsy; intermittently asking the awake patient about numbness or tingling in the little finger will help avoid this complication. Entry of the dissecting instrument into the palmar fat space ensures sufficient distal decompression. A Senn retractor can be employed to provide upward traction on the proximal portion of the incision, and further division of the TCL 1-2 cm proximal to the wrist crease into the deep fascia of the forearm ensures complete proximal decompression (Fig. 199-5). The most common cause of surgical failure is incomplete division of the proximal aspect of the ligament.42 Adequate release of the canal may be confirmed by freely passing the no. 4 Penfield dissector under the wrist and gently palpating both proximally and distally for any residual retinaculum (Fig. 199-6).
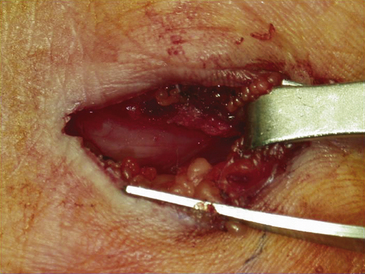
FIGURE 199-6 The median nerve has been freely released distally as well by this mini–carpal tunnel release procedure.
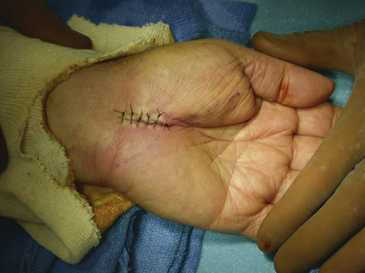
Inspection of the median nerve, which may appear hyperemic, and exploration of the carpal tunnel for evidence of mass lesions or anatomic variants is indicated. There is no proven benefit to separately decompressing the motor branch of the median nerve and numerous studies have shown no improved outcome with routine performance of internal neurolysis of the median nerve during a virgin procedure; in the setting of recurrent disease and repeat surgery in which fibrosis and scarring is prevalent, there may be a role for neurolysis.43 Once the wound has been thoroughly irrigated with sterile saline and meticulous hemostasis is obtained with bipolar coagulation, the fascia and subcutaneous tissues may then be reapproximated with several 3-0 interrupted, inverted Vicryl sutures (Ethicon, Inc., Somerville, NJ). The skin is closed with interrupted or simple running 4-0 nylon sutures; alternatively, the wound may be closed in a single layer of interrupted vertical mattress sutures with a nonabsorbable monofilament suture material (4-0 nylon) (Fig. 199-7). A bulky soft hand dressing is applied, the wrist is not immobilized with a splint, and the entire procedure should be completed within 15 to 30 minutes.
The standard CTR is associated with good to excellent relief of symptoms in 80% of patients, partial relief in 10%, no change in 9%, and worsening of symptoms in 1%.16,18,44 A reduction in pain and numbness of the affected hand is usually the first improvement noted by the patient, often immediately following the release. In a recent study involving 188 CTR procedures, objective measurements of sensory discrimination and motor function demonstrated normal levels of function in 60% and 92% of patients, respectively; a review by Gellman and others revealed improvements in grip and pinch strengths by 12 weeks after surgery.45–48 Several prospective studies have also shown similar improvements in conduction velocities and distal latencies, some as soon as 2 weeks postoperatively, as measured by electrodiagnostic testing.49–51 Complications of the procedure include painful neuroma of the palmar cutaneous nerve, section of the motor branch with subsequent weakness, postoperative fibrosis and hypertrophic or tender scarring, poorly understood pillar pain, wound infections and bow-stringing of the flexor tendons. Patient-related factors that portend the likelihood of unsatisfactory outcomes include systemic metabolic disease (diabetes mellitus, obesity, etc.), severe muscle weakness at the time of surgery, and a lack of response to corticosteroid injections, if attempted, before the operation.51,52
Modifications of the traditional open CTR include the technique described by Paine and Polyzoidis, in which a 1.5-cm transverse incision is made in the wrist crease prior to using the Paine retinaculatome.53 The retinaculatome consists of a small knife that extends perpendicular to a thin footplate, which is passed under the ligament in proximal and distal directions to blindly transect the flexor retinaculum.
Endoscopic (Closed) Carpal Tunnel Release
Endoscopic surgical release of the carpal tunnel has gained popularity in the last decade and both single-portal and biportal techniques have been described. Since its introduction in 1989, six types of endoscopic procedures for the treatment of CTS have been developed, three using a single incision (uniportal) and three using two small incisions (biportal). Chow, Brown, and Okutsu have each reported a large series with excellent results.54–57 The surgery may be done under local anesthesia but many surgeons prefer intravenous sedation and at least a laryngeal mask airway. In a biportal method known as the Brown technique, a tourniquet is applied and a 1-cm incision is made 1-2 cm proximal to the distal wrist crease ulnar to the palmaris longus tendon. The underlying antebrachial fascia is bluntly divided and a synovial elevator is placed distally beneath this fascia and into the carpal tunnel. The synovium is removed from the undersurface of the TCL and an obturator with a slotted cannula is inserted through the carpal tunnel and out approximately 4 cm distal to the distal wrist crease along the third web space. Following removal of the obturator, a 30-degree rigid endoscope is inserted distally and the undersurface of the TCL may be visualized through the slotted end of the cannula. A hook blade is inserted proximally and advanced to the distal end of the flexor retinaculum. The ligament is then divided completely from distal to proximal on the ulnar side of the ligament under direct visualization; this may require more than one pass. It is not recommended that the surgeon attempt to inspect or explore the median nerve. The tourniquet is then deflated, hemostasis is achieved, and the incisions are closed with three simple nylon sutures. A volar splint is applied for the duration of 5 to 7 days and patients typically return to work within 2 to 4 weeks following the procedure.
Reports have demonstrated no difference in long-term outcomes between the open and closed approaches.47,58 A randomized trial performed by Agee et al. showed equal outcomes but faster initial recovery in patients undergoing endoscopic release compared with the traditional open approach.59 Relative contraindications to the endoscopic surgery include rheumatoid arthritis, significant tenosynovitis, recurrent CTS, concurrent ulnar tunnel syndrome or a space-occupying lesion. Many peripheral nerve surgeons who have become very comfortable with the traditional open approach are reluctant to incur the greater expense, steep learning curve, and higher number of serious complications associated with endoscopic carpal tunnel release.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree