Complications of osteoporosis in spine surgery
Subsidence
Pedicle fracture
Proximal junctional kyphosis
Compression fracture
Pseudoarthrosis
Implant loosening/haloing
15.3 Preoperative Evaluation and Medical Management
Preoperative evaluation of the patient with suspected osteoporosis includes a DEXA scan and metabolic labs (vitamin D, parathyroid hormone, and calcium). These tests are important in determining the extent of osteopenia or osteoporosis and therefore aid in preoperative planning. Currently, a dual-energy X-ray absorptiometry scan (DEXA) for bone mineral density measurement is considered the gold standard for osteoporosis diagnosis. A DEXA value of < −1 to > −2.5 is considered osteopenic, and a level < −2.5 is considered osteoporotic. Some surgeons advocate not operating on severely osteoporotic patients due to the increased risk, though set cutoffs for avoiding surgery have not been determined [17]. Despite the importance of the osteoporotic spine on results of spinal fusion, only 44 % of surgeons in one study ordered preoperative DEXA scan and 12 % ordered vitamin D and calcium levels prior to considering instrumented fusion [19].
Before we discuss medical treatment of osteoporosis, we need to understand the normal bone growth. Typical bone mainly consists of osteoblasts, osteocytes, and osteoclasts. Osteoblasts cells are bone-forming cells, while osteoclasts are responsible for bone resorption. In normal bone, remodeling of bone is a continuous constant process [63]. Osteoporosis develops when there is an imbalance between bone resorption and bone formation. This imbalance can be caused by three mechanisms: inadequate peak in bone mass during skeleton growth, excessive osteoclastic bone resorption, and insufficient new bone formation response during bone remodeling [63].
Two types of osseous tissue form bone: trabecular bone and cortical bone. Trabecular bone (cancellous bone) is the soft, spongelike bone in the periphery of long bones and vertebrae. Cortical bone (compact bone) is the dense, hard outer layer of bones and the middle of long bones. Trabecular bone has a greater surface area for metabolic activity than cortical bone; therefore, it is more affected in osteoporosis. This explains why wrist, hip, and spine (bones with relatively high trabecular bone) are common sites of osteoporotic fractures.
Several pharmacologic treatments are used in treatment of osteoporosis or low bone density. American College of Physicians (ACP) recommends that clinicians choose among drugs on the basis of risks, benefits, and adverse effects in individual patients [62]. Secondary causes of osteoporosis must be excluded before commencement of medical treatment. Alcoholism, multiple myeloma, osteomalacia, use of glucocorticoids, and medical illnesses such as rheumatoid arthritis need to be first excluded as these conditions would require specialized management in addition to standard medical management of osteoporosis [17].
Bisphosphonates act through osteoclast inhibition, reducing bone turnover. They are synthetic analogs of pyrophosphate which bind to hydroxyapatite in bone remodeling, hence reducing bone resorption activity of osteoclasts. Bisphosphonates drug class includes alendronate, etidronate, ibandronate, pamidronate, risedronate, and zoledronic acid. All of them, except etidronate and pamidronate, are approved by Food and Drug Administration (FDA) for osteoporosis treatment. Because of the strong evidence of bisphosphonates in effectively reducing the risk for vertebral, nonvertebral, and hip fractures, they are considered as first-line treatment of osteoporosis [62]. Alendronate and risedronate have been studied more than drugs in the class. Alendronate (70 mg once weekly or 10 mg daily) is the first-line option in treatment of osteoporosis. Risedronate (35 mg once weekly or 5 mg daily) is an alternative choice in case of alendronate intolerance. Zoledronic acid, with alendronate and risedronate, lies in the first-line in osteoporosis treatment [79]. Alendronate is also considered the first-line in the treatment of steroid-induced osteoporosis. However, it is not FDA approved in prevention of steroid-induced osteoporosis. Risedronate is considered the first-line in prevention of steroid-induced osteoporosis.
Parathyroid hormone (PTH) is another strategy for osteoporosis treatment. The mechanism of action of PTH in producing net bone formation is complex and not completely elucidated. The extent to which these drugs impact fusion is largely unknown. Animal studies have demonstrated that bisphosphonates appear to impede fusion mass, but human studies demonstrate increased fusion mass radiographically, though clinical outcome was not affected [31]. To date, PTH has not been studied in humans regarding its potential to improve fusion (Table 15.2). In animal studies, however, it has been shown to improve the fusion rate and fusion mass [31]. In light of the complications associated with osteoporosis and fusion, it may be prudent to consider delaying surgery in patients with osteoporosis when possible and allowing for treatment in order to improve bone quality. However, the absolute cutoff for avoiding surgery has not been defined, and there is no definitive evidence that treatment of osteoporosis prior to spine surgery improves outcomes [17, 32].
Table 15.2
Initial diagnostic evaluation of osteoporosis
Initial diagnostic evaluation of osteoporosis |
|---|
Dual-Energy X-Ray Absorptiometry (DEXA) Scan (T-score < −2.5 = osteoporosis) |
Calcium level |
25-hydroxy vitamin-D |
Comprehensive metabolic panel |
Complete blood count |
More recently, two agents have been introduced for the treatment of osteoporosis: denosumab, which is a monoclonal antibody that inhibits the activation and differentiation of osteoclasts, resulting in less bone resorption, and teriparatide (recombinant parathyroid hormone 1–34), which in contrast directly stimulates bone growth [46]. Both have been found to reduce vertebral fracture risk in postmenopausal women with osteoporosis [46]. However, their effects on patients undergoing spinal deformity correction are unknown. A recent prospective cohort study found that teriparatide was more effective than combined vertebroplasty and anti-resorper agent for treating post-vertebroplasty new-onset adjacent vertebral compression fractures [73].
Denosumab is a new drug recently approved by FDA in 2010. It is fully human monoclonal antibody that targets the receptor activator of nuclear factor-κB ligand (RANKL) that blocks its binding to RANK, inhibiting the differentiation and activity of osteoclasts. Denosumab is considered as first-line agent in osteoporosis treatment [79].
FREEDOM, a randomized clinical trial, included 7,868 postmenopausal women with osteoporosis found that denosumab (60 mg once every 6 months) for 36 months was associated with a reduction in the risk of vertebral, nonvertebral, and hip fractures [14]. The FREEDOM trial was extended for up to 10 years. First 2 years results (represents 5 years since FREEDOM study commencement) showed further increase in bone density at the lumbar spine and total hip [56].
The DECIDE trial compared the efficacy and safety of denosumab with alendronate in 1,189 postmenopausal women with low bone mass. Denosumab achieved better results in both bone density and bone turnover reduction compared with alendronate and similar safety profile [8]. Another trial showed more adherence and compliance of patients receiving denosumab than those taking alendronate [27].
Mixed treatment comparison in a recent meta-analysis showed that denosumab is more effective than alendronate, risedronate, and other drugs in preventing new vertebral fractures [26].
Teriparatide is the only FDA-approved anabolic drug for osteoporosis treatment. Preotact, a new anabolic agent, is pending FDA approval. Teriparatide (20 μg/day) has been proved to decrease both spine and vertebral fractures but hip fractures in postmenopausal women with history of previous vertebral fractures [55, 61]. The manufacturers of teriparatide, Eli Lilly and Company, state in the insert package that teriparatide treatment should not exceed 2 years [20]. This is based on osteosarcoma cases developed in rats treated with teriparatide for 2 years [75, 78]. However, osteosarcoma was not reported in clinical studies of humans taking teriparatide. The Osteosarcoma Surveillance Study group have recently published the findings of the first 7 years of that ongoing 15 years study evaluating the potential association between teriparatide and the development of osteosarcoma in humans [3]. Interestingly, there were no osteosarcoma patients who had a prior history of teriparatide treatment.
Teriparatide increases bone density at most sites and decreases nonvertebral fractures compared to alendronat [6]. Additionally, teriparatide is superior to alendronate for treating glucocorticoid-induced osteoporosis [67]. Moreover, case reports show that teriparatide is effective in treatment of alendronate-associated osteonecrosis of the jaw [11, 29, 41, 43]. However, cost considerations and lack of studies showing hip fracture reduction prevent using teriparatide as a first-line agent.
Combination of teriparatide and alendronate is not recommended. Combination treatment is not more effective than either agent alone [5, 22]. Moreover, alendronate decreases the effect of teriparatide to increase bone density and turnover in both men and women [23, 24].
Calcitonin, which had previously been employed, is no longer considered appropriate therapy for osteoporosis [46].
In 2010, the American Association of Clinical Endocrinologists (AACE) published guidelines and recommendations for diagnosis and treatment of osteoporosis [79]. Based on level of evidence, they generated the following recommendations for choosing drugs in osteoporosis treatment:
First-line agents: alendronate, risedronate, zoledronic acid, denosumab
Second-line agent: ibandronate
Second- or third-line agent: raloxifene
Last-line agent: calcitonin
Treatment for patients with very high fracture risk or in whom bisphosphonate therapy has failed: teriparatide
Recommendation against the use of combination therapy.
15.4 Surgical Strategies for the Osteoporotic Spine
In light of the significant challenges in the surgical management of osteoporotic bone, multiple surgical strategies aimed at improving pullout strength, augmenting fusion, and reducing complications have been employed. Among these are expandable pedicle screws, polymethyl methacrylate (PMMA) augmentation, cannulated screws filled with PMMA, increased levels of fixation, bicortical screw purchase, dual threaded screws, and less rigid implants, among others (Table15.3).
Table 15.3
Summary of surgical techniques for managing the osteoporotic spine
Surgical strategies for managing osteoporosis |
|---|
Expandable pedicle screws |
Polymethylmethacrylate (PMMA) augmentation |
Cannulated screws filled with PMMA |
Increased levels of fixation (including pelvic fixation) |
Bicortical screw purchase |
Dual-threaded pedicle screws |
Less-rigid implants |
Biomechanical data suggests that pedicle screws that expand within the pedicle may substantially improve pullout strength in bone compromised by osteoporosis [13, 48]. Early work by Cook et al. reported that 86 % of patients with osteoporosis who underwent expandable pedicle screw placement experienced fusion, with no instances of screw pullout or loosening [13]. In a preliminary study, Wu et al. described 125 consecutive patients with severe osteoporosis who underwent placement of expandable pedicle screws. They also noted no instances of screw loosening or pullout, and patients experienced significantly improved outcomes as measured by Japanese Orthopedic Association (JOA) scores and visual analog scale (VAS) scores [80]. In another comparison of expandable pedicle screws with conventional pedicle screw constructs in the treatment of patients with osteoporosis who underwent lumbar spine fusion demonstrated that in 80 patients who received expandable screws, there was a 92.5 % fusion rate compared to 77 patients who underwent conventional pedicle screw placement who demonstrated an 80.5 % fusion rate. This result was statistically significant [81]. Furthermore, in the same study, screw loosening occurred in a significantly lower percentage (4.1 %) of screws placed in the expandable group compared to 12.9 % of screws placed in the conventional group [81]. Furthermore, JOA and Oswestry Disability Index (ODI) scores were significantly better compared to preoperative scores in the expandable pedicle screw group [81]. A biomechanical comparison of expandable pedicle screws and PMMA-augmented pedicle screws in osteoporotic cadavers suggested that both expandable screws and PMMA-augmented screws exhibited significantly enhanced stability as compared with conventionally placed screws [48]. The problem with expandable pedicle screws, however, is revision surgery. Typically, these implants destroy the pedicle if removal is necessary for any reason.
Polymethyl methacrylate has been used with increasing frequency to augment the fusion constructs in osteoporotic patients. Several studies have demonstrated the utility of PMMA in increasing pullout strength and improving fixation [16, 38, 48, 52, 59, 68, 84]. Biomechanical data in cadavers suggests that PMMA-augmented pedicle screws provide superior screw stability as compared to conventional pedicle screws, and this fixation is comparable to that of expandable pedicle screws [48]. In one cadaver study, as bone mineral density decreased, PMMA-augmented screws demonstrated significantly stronger pullout strength as compared to bicortically placed conventional pedicle screws at S1 [84]. In a 3-year follow-up study of 37 osteoporotic patients undergoing pedicle screw placement with PMMA augmentation, Moon et al. found that VAS scores for back and leg pain were significantly reduced from 7.87 to 2.30 and 8.82 to 1.42 (p = 0.006), respectively [54]. Further demonstrating the clinical utility of PMMA-augmented pedicle screws, Sawakami et al. showed a significant decrease in haloing around PMMA-augmented screws (29.4 % vs. 71.4 %) and a significantly higher fusion rate (94.1 % vs. 76.1 %) [68]. Additionally, PMMA augmentation has been found to be useful in anterior approaches as well [38]. A study of 62 osteoporotic patients who underwent ALIF with or without PMMA augmentation and were followed for over 2 years demonstrated that those who had PMMA augmentation demonstrated significantly less graft subsidence (5.2 % vs. 19.6 %, p = 0.001). Furthermore, the vertebral body height at the index level was significantly higher in the PMMA group (10.7 % vs. 3.9 %, p = 0.001) [38]. Another option for PMMA-augmented fusion for minimally invasive surgery is pedicle screw placement with cannulated screws through which PMMA is injected. A prospective study of this technology in osteoporotic patients over the age of 70 with a mean follow-up of 20–49 months demonstrated that no radiographic or clinical cases of nonunion were observed and that pain and function were improved at 6 months and maintained at final follow-up [59]. Additionally, there was no evidence of cement leakage, a known complication of PMMA-augmented screws [17, 59]. However, a comparison of standard screws with PMMA augmentation and screws with cannulated PMMA augmentation in a synthetic vertebral body revealed greater pullout strength in the standard screw group [10]. This has not been corroborated clinically, however. One concern with PMMA screw augmentation is that vertebrectomy may be necessary if the PMMA becomes infected postoperatively.
In addition to expandable pedicle screws and PMMA-augmented screws, other surgical techniques for the osteoporotic spine have been advocated to reduce the complications associated with osteoporotic bone in spinal fusion. One such technique includes the application of Nesplon tape in either the sublaminar or sub-pars space connected to a rod. One study of this technique demonstrated that tape applied in this manner in cadaver specimen resulted in significantly stronger fixation and a stiffer construct when compared to pedicle screw constructs alone [28]. This may be due to the higher regional bone density concentration in the lamina as compared to the pedicle. Evidence suggests that the insertional torque required to place a pedicle screw is positively correlated with the patient’s bone mineral density [42]. Because of this, knowing the bone density prior to surgery may influence the number of levels needed for fusion in osteoporotic patients [42]. Some authors advocated adding multiple levels of fusion in the osteoporotic spine, with routine extension to the pelvis for lumbosacral fixation in patients with osteoporosis [17]. Also, same-diameter tapping prior to pedicle screw placement was shown to result in decreased insertional torque and thus pullout strength, and therefore, undertapping or not tapping prior to pedicle screw insertion has been advocated [17] (Fig. 15.1).
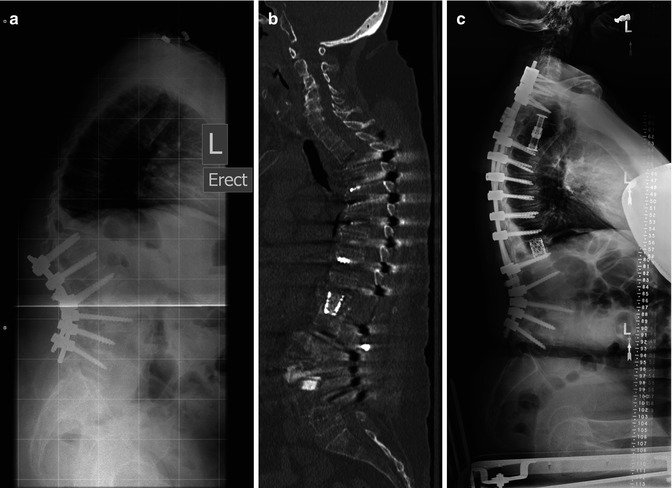

Fig. 15.1
(a) Lateral X-Ray demonstrating a T11 fracture and kyphotic deformity following L2 pedicle subtraction osteotomy in a 59 yo woman with a history of chronic steroid use for Lupus and osteoporosis. (b) Sagittal CT reconstruction in the same patient demonstrating a T3-4 compression fracture with resultant kyphosis and progressive paraparesis following T11 vertebral column resection and extension of fusion to T3. (c) Lateral scoliosis X-Ray in the same patient following T3-4 vertebral column resection and extension of fusion to C7
15.5 Vertebroplasty/Kyphoplasty for Osteoporotic Fractures
Osteoporotic vertebral fracture is a significant cause of pain and disability in the elderly [50, 65]. The incidence of osteoporotic vertebral fracture is likely to increase as the population ages. Recently, vertebroplasty and kyphoplasty have been employed to treat both the pain and deformity associated with these fractures [40, 50, 65]. Their use has been increasing at a rapid rate [40]. Vertebroplasty is meant to reduce the pain of fractured vertebrae and prevent worsening of vertebral body height loss by direct pedicular injection of PMMA. Kyphoplasty, on the other hand, uses an expandable balloon to try to reverse the kyphosis caused by the fracture and create space for PMMA to be injected in order to address both the pain and deformity associated with vertebral compression fractures. A recent study questioned the efficacy of vertebroplasty in the management of osteoporotic compression fractures [34]. In a randomized trial of 131 patients with one to three levels of vertebral body fracture, 68 patients underwent vertebroplasty and 63 underwent sham injections of local anesthetic. At 1 month and 3 months, there was no significant difference in outcomes between the vertebroplasty and control group [34]. However, there was a significant trend toward a clinically meaningful result (defined as a 30 % reduction in pain) in the vertebroplasty group (p = 0.06). Also, there was no control group who received only medical management, and there was significant crossover from the control group to the vertebroplasty group at 3 months (51 % vs. 13 %) [34]. In contrast, a randomized controlled trial of 80 patients comparing vertebroplasty vs. optimal medical management of vertebral compression fractures in osteoporosis noted that the vertebroplasty group experienced significantly improved VAS scores at 1 week that persisted over 36 months as well as improved quality-of-life (QoL) scores that persisted at 36 months compared to the control group [21]. Similarly in another randomized controlled trial of vertebroplasty and maximal medical therapy that included 202 patients, there was a significant decrease in VAS pain scores at 1 month that persisted at 1 year [39]. These prospective studies suggested that vertebroplasty is effective and durable in the treatment of osteoporotic vertebral fractures. Further work has demonstrated that this may be the case in the very elderly as well. DePalma et al. prospectively studied vertebroplasty for osteoporotic vertebral compression fractures in 123 consecutive nonagenarians and found that mean VAS scores decreased significantly from 7.6 preprocedure to 3.1 at 30 min following the procedure, 1.2 at 1 month, and 0.5 at 2 years, respectively [16].
Kyphoplasty has not been studied to the same degree as vertebroplasty. However, studies demonstrated its potential value [25, 49, 71, 82]. A study of 26 patients undergoing kyphoplasty for osteoporotic vertebral compression fractures demonstrated a statistically significant reduction in VAS scores from 7.7 to 3.1 and 2.9 at 1 day and 3 months following the procedure [49]. Additionally, sagittal Cobb angle was significantly reduced from 18.5 degrees before the procedure to 9.2 degrees after (p < 0.001) [49]. Mirroring this result, a study of kyphoplasty in 27 fractured vertebrae in 25 patients noted a significant reduction in Cobb angle (17.18 degrees to 9.35 degrees, p < 0.05). Furthermore, anterior and medial vertebral body heights were increased by 33 and 50 %, respectively [82]. Evidence suggested this improvement in vertebral body height and Cobb angle was sustained at 12 months [25]. In a prospective study of 40 kyphoplasty patients with 1-year follow-up, anterior and medial vertebral body height were increased by 51.25 % and 52.29 %, respectively, with no loss at 1 year [25]. Additionally, scores on the VAS, North American Spine Society scale, and Short Form (SF)-36 scores improved significantly at 1 year [25].
Direct comparison of vertebroplasty and kyphoplasty in a randomized controlled fashion has not occurred. However, a review of the literature on these treatments demonstrated that both were more efficacious at reducing pain and improving mobility in the short-term compared to conservative therapy alone [7].
Despite the relative safety of vertebroplasty and kyphoplasty, complications have been reported. The main complications include cement leakage, cement embolism, and adjacent level fracture [25, 44, 47]. One analysis of patients who experienced vertebral fracture after kyphoplasty noted that 12/14 (86 %) occurred within 6 months of the vertebroplasty and that 10/14 (71 %) of the fractures occurred at the adjacent level, raising the question of the effect of vertebroplasty on fractures at adjacent levels [47]. However, other studies have noted low levels of adjacent fractures and that many of the fractures would have occurred anyway and were related to the degree of osteoporosis [53]. In some studies, the adjacent fracture rate was lower in those treated with vertebroplasty [21]. A meta-analysis of complications associated with vertebroplasty and kyphoplasty concluded that when analyzing all studies as well as only prospective studies, vertebroplasty was found to have increased procedure-related complications including symptomatic and asymptomatic cement leakage [44]. Future prospective studies are necessary to corroborate this analysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








