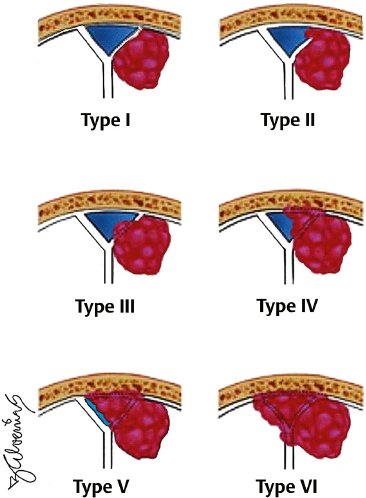
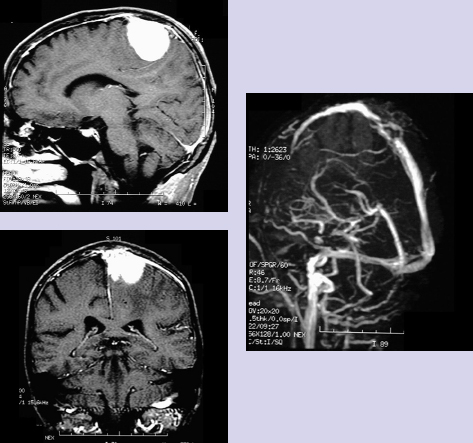
Chapter 2 Case A 40-year-old right-handed woman presented with headache and a new onset of generalized seizures. A physical examination showed mild left hemiparesis. Participants Combined Surgical and Radiosurgical Treatment of Parasagittal and Falx Meningiomas with Superior Sagittal Sinus Invasion: M. Necmettin Pamir, Selcuk Peker, and Koray Özduman Total Removal of Parasagittal Meningiomas Involving the Superior Sagittal Sinus: Marc P. Sindou, P. Hallacq, and Jorge E. Alvernia Moderators: Management of Parasagittal Meningiomas Involving the Superior Sagittal Sinus: Partial Removal with Radiosurgery vs. Total Removal with Repair: Kenji Ohata and Rahadian Indarto Susilo This patient’s neurologic examination showed mild hemiparesis, and magnetic resonance imaging (MRI) demonstrated a left-sided parasagittal meningioma located in the middle third. Magnetic resonance venography showed partial occlusion or narrowing of the superior sagittal sinus (SSS). Our recommendation for this patient is surgical resection of the tumor outside the sinus and subsequent radiosurgery for the residual portion that invades the sinus. Parasagittal meningiomas invading the SSS have long presented a problem for neurosurgeons. Most of the patients with this lesion have neurologic signs or symptoms, prompting some form of treatment, and even if asymptomatic, most of these tumors grow over time, infiltrating surrounding structures. But choosing the form of treatment is not so straightforward. The literature from the microsurgical era concentrates on two treatment options: conservative resection, and radical resection of both the tumor and the invaded sinus combined with reconstruction of the sinus. However, accumulating evidence has shown us that neither of these options provides a safe, effective, and long-lasting solution to the problem, and this lack of a solution has prompted a search for new and more effective treatment options. Parasagittal and falx meningiomas make up a significant proportion of intracranial meningiomas, with reported rates ranging from 19.24 to 33.7%.1–4 In early studies, these meningiomas were classified based on whether or not they showed hyperostosis. An anatomic classification into anterior, middle, and posterior locations was first devised by Olivecrona.4 Anterior cases are located between the crista galli and the coronal suture, middle cases arise between the coronal and lambdoid sutures, and posterior cases are localized between the lambdoid suture and the torcular. Most of these lesions occur in the middle third, and the reported relative rates are 14.8 to 33.9%, 44.8 to 70.4%, and 9.2 to 29.6% in the anterior, middle, and posterior portions, respectively.1,5–8 Superior sagittal sinus involvement is reported in a significant proportion of the cases, with Simpson9 noting a rate of 40%. The extent of venous involvement by the meningioma can range from invasion of the outer surface of the venous wall to complete invasion and obliteration of the sinus. Several authors have devised classification schemes for surgical decision making. The first detailed classification scheme of Krause was later modified by Merrem and then Bonnal and Brotchi.10 This widely cited classification scheme was in turn later modified by Hakuba to include eight subtypes. The latest, simplified version by Sindou and Alvernia11 describes six types of parasagittal meningiomas classified according to the degree of sinus invasion: Type I: Invasion of the outer surface of the sinus wall Type II: Invasion of the lateral recess Type III: Invasion of the lateral wall Type IV: Invasion of the lateral wall and roof Type V: Total occlusion of the sinus with one wall free of tumor Type VI: Total occlusion of the sinus without one wall free of tumor The reported rates of these types are 31%, 8%, 11%, 13%, 5%, and 32%, respectively.11,12 The anatomic classification into three segments along the anteroposterior axis is not merely of diagnostic significance but relates to the neurologic consequences of SSS closure at that segment. According to their terminal drainage, the superficial veins of the cerebral hemispheres are divided into superior sagittal, falcine, sphenoid, and tentorial groups.13–15 These systems are interconnected by three large anastomotic veins: the vein of Trolard, the vein of Labbé, and the superficial sylvian vein. The superior sagittal group drains blood from both hemispheres into the SSS, the largest draining vein. The size of the SSS increases from anterior to posterior with the addition of veins from the frontal, parietal, and occipital lobes.13–15 The terminal veins adjoin to form 1- to 2-cm free venous segments along the superior margin of the hemisphere in the subdural space. These terminal cortical veins can drain directly into the SSS or pass through lacunae before they drain into the sinus.14 The clinical consequences of acute occlusion, thrombosis, or sacrifice of the SSS were first documented in the first quarter of the 20th century,13 and it was soon concluded that the site of closure is the dominant factor in the patient’s neurologic outcome. Sacrifice of the anterior third is well tolerated, but some authors note that general slowing of the patient’s thought process and activity, or even akinetic mutism, are reported after such sacrifice, although these phenomena are rare.15 Sacrifice of the middle third causes hemiplegia, more prominent in the lower extremities, and akinesia.15 The posterior third is the largest portion and receives the straight sinus, and acute occlusion or surgical sacrifice of this portion carries a significant risk of fatal brain edema and increased intracranial pressure. Although acute obliteration of the sinus is associated with such serious consequences, a more gradual closure during tumor growth is usually well tolerated. One must bear in mind that the gradual closure from tumor invasion is not merely a closure of the sinus. The living organism reacts actively to changes in the “interior milieu,” and gradual occlusion of the sinus is certainly accompanied by functional changes in venous drainage. Closure of the main drainage route does not necessarily lead to diversion of the venous drainage to other systems away from the SSS. In many cases, venous collaterals take over the role of the main channel, and sacrifice of these collaterals leads to morbidity similar to that of SSS sacrifice. Whether, or how much, the tumor contributes to venous drainage of the region is not known.16 The goal in managing this type of “problem” meningioma is clear: keep the patient fully functional and prevent or provide long-term relief from problems associated with intracranial tumor growth. The availability of several alternative treatment possibilities indicates that there is still no single best form of treatment for meningiomas invading the SSS. The most straightforward treatment option is complete surgical excision. Meningiomas located in the anterior third are the least controversial with regard to treatment, and most authors recommend simple radical resection. Parasagittal meningiomas located in the middle third of the SSS are the most difficult to treat because of the abundance of afferent veins, the significant morbidity associated with their sacrifice, and the high risk of recurrence. Meningiomas in this group were early christened as “problem” meningiomas.2 Total resection is technically demanding; it can be associated with significant morbidity in patients with invasion of the SSS. In some cases total resection is not possible. The general consensus for lesions that have limited sinus invasion is surgery followed by limited local reconstruction. Simple repair or patching with endogenous material (for example, muscle grafts) is adequate in most of these cases. If the invasion is more extensive, a radical excision necessitates venous reconstruction, but this entails a significant complication rate. Some authors have advocated more conservative resections to avoid this increased risk, but conservative approaches are associated with continued growth. Authors who have advocated subtotal resection of these meningiomas resect the tumor outside the sinus and leave the invading portion untouched.3,17 Surgery is planned to restore or preserve function in an attempt to combine the lowest possible risk with the maximum benefit to the patient, based on the notion that most meningiomas are slow-growing tumors and even subtotal resection provides long, progression-free periods. However, most of these patients survive for long periods and therefore regrowth is inevitable after such conservative resections.18–20 Recurrences are managed through repeated conservative resections until the sinus is obliterated, when total resection becomes an option. Although this idea may sound attractive, it is well known that the efficacy of surgery decreases and the complication rate increases with each repeated surgical intervention. The literature contains no solid scientific evidence on the long-term results of subtotal resection. The high rate of regrowth after subtotal resection and the fact that complete surgical resection including the invaded structures is associated with lower rates of recurrence led several authors to develop techniques to resect the invaded sinus along with the tumor. As noted above, resecting part of a patent SSS can cause serious morbidity and mortality due to venous infarction and brain edema. Therefore, techniques have been developed to repair or reconstruct the sinus after the portion invaded by the meningioma is removed.5,6,10–12,21–29 Most recent studies, however, indicate that such aggressive approaches are not necessarily associated with good surgical results or low recurrence rates, and they are still associated with significant morbidity.25 Such a venous reconstruction is a formidable surgical challenge, and the literature contains only a few large series of SSS reconstruction totaling fewer than 200 cases. Bonnal and Brotchi10 were the leading authors advocating such procedures. In 1978, they published the results of 34 patients with SSS repair or reconstruction with venous allografts for parasagittal meningiomas. In nine patients, the surgeons were able to preserve the patency of the sinus without using a graft. In the other 25, they removed one or more walls of the SSS and then rebuilt the structure using a dural or venous graft. In one case, they needed to remove the entire SSS and then create a new sinus structure using a total vein graft. Hakuba23 also reported his results with 23 cases of para-sagittal meningiomas. In six patients, he totally removed the tumor and the sinus. Seventeen patients had sinus involvement and, after total tumor excision, Hakuba repaired the sinus wall or reconstructed the sinus with a vein graft. In this group, 29% of patients had postoperative paresis. On two occasions, Sindou and his colleague11,12 reported their experience with the aggressive management of para-sagittal meningiomas invading the SSS. The second study, of 100 meningiomas that invaded the dural sinuses, included 92 cases that were located in the SSS. Of these, 30.4% were in the anterior third in close relation to the precentral veins, 52.3% were in the middle third in relation to the postcentral veins, and 17.4% were in the posterior third.11 The authors reported gross total removal in 93% of these patients (Simpson grade I or II) and radical excision combined with coagulation of a small amount of residual tumor (Simpson grade III) in the other 7%. The permanent neurologic morbidity rate was 8%, and the mortality rate was 3%. Looking back at those publications, one realizes that the techniques are not very efficient. In Bonnal and Brotchi’s series, the immediate postoperative control angiogram showed a patent SSS in 87% of the 34 patients. This rate was 66% in Hakuba’s23 series and 64% in Sindou’s12 2001 series, indicating that, in up to a third of cases, the venous reconstruction did not work. Similarly, the recurrence rates after radical resection are not very impressive either. Regardless of the form of surgical treatment, recurrence is common in patients with meningiomas invading the SSS. This high risk of recurrence was originally documented by Simpson’s9 landmark study, which indicated a recurrence rate of 5% for Simpson grade I and 17% for Simpson grade II resections. This is most likely due to microscopic residuals undetected at the time of surgery. Mathiesen and colleagues30 reported microscopic residual meningioma growth in dural resection margins in 41% of “radical” Simpson grade I operations. Authors who have not attempted sinus reconstruction have reported similarly high rates of recurrence in patients in whom radical resections were possible. Jääskeläinen31 reported a recurrence rate of 21% after seemingly complete removal. At a mean follow-up of 25.4 years, Caroli and colleagues5 reported a 9.3% recurrence rate after Simpson grade I and a 42.9% recurrence after Simpson grade II resections. The mean time to recurrence was 6.8 years after grade I removal and 4.7 years after grade II or III resections. These results indicate that a more radical resection can decrease but not eliminate the risk of recurrence in patients with parasagittal meningiomas invading the SSS. One can also conclude that a more radical resection does not significantly delay the time to recurrence either. Based on these findings, it is not surprising that, even in the hands of masterful surgeons, radical resection combined with SSS reconstruction are associated with considerable rates of recurrence. In 2003, Brotchi’s research group25 documented the long-term results for these cases. Of the 25 individuals who underwent partial or total SSS removal and sinus reconstruction, 15 had total tumor excision, and these patients had been followed for more than 10 years. Five of these 15 individuals (33%) developed a focal meningioma recurrence. The remaining 10 of the 25 patients underwent subtotal tumor excision, and eight of them had been followed for more than 10 years. Five of these eight patients (63%) developed local recurrence. Because of these outcomes, the authors questioned the efficacy of their approach and concluded that the optimal strategy for patients with meningiomas involving the SSS is gross tumor removal followed by monitoring, and radiosurgery if regrowth occurs. In stark contrast, Sindou and Alvernia11 reported a recurrence rate of 4% over a mean 8-year follow-up period (3–23 years). Such perfect results have not been replicated by any other master surgeon. Having analyzed the literature, we gave up the strategy of radical resection and reconstruction and started using a combination of surgery and the gamma knife.8 Our rationale is based on our previous good results with the combination of surgery and radiosurgery for meningiomas in other locations32 and the good results of other groups with this type of meningioma.7 The high complication rates of aggressive treatment strategies and the risk of recurrence after conservative treatment have created a need for alternative treatment strategies. Radiosurgical treatment of meningiomas has proven safe and effective, and this treatment modality for meningiomas has become very popular in recent years for the primary treatment of small meningiomas or as an adjuvant for residual or recurrent cases.26,27 The reported tumor growth control rates range from 85 to 95%.27 As in the case of cavernous sinus meningiomas, this relatively less-invasive treatment modality can potentially be used as an adjunct or alternative in patients with parasagittal meningiomas invading the SSS to achieve long-term control of tumor growth with little morbidity.28 Currently there is no definitive evidence to show the superiority of aggressive or less invasive treatment paradigms. Only two studies have been published to test the hypothesis that the gamma knife can be used effectively to treat parasagittal meningiomas that invade the SSS. Kondziolka and colleagues7 conducted a multicenter study and documented treatment results for 23 such patients. Most of these meningiomas had invaded the middle or posterior region of the SSS, and the mean tumor volume was 10 cm3. Seventy-eight patients underwent radiosurgery as the primary therapy, with a 5-year actuarial tumor control rate of 93%, and none of the tumors smaller than 7.5 cc showed regrowth in the long term. For the 125 patients who had undergone surgery before gamma knife treatment, the 5-year control rate was only 60%. The authors reported that most cases of radiosurgery failure were due to remote tumor growth, as a precise definition of meningioma borders can be complicated in patients who have undergone previous surgery for SSS-invading meningiomas. In the 203 cases reported by Kondziolka and colleagues, the median marginal dose was 15 Gy. Sixteen percent of the patients developed symptomatic edema after radiosurgery. The authors analyzed the potential causes of this edema and found that the only factor correlated with this complication was previous neurologic deficit. In all cases, the edema resolved with medical therapy. The authors concluded that, in patients with a small meningioma (< 3 cm diameter) that has invaded the SSS but in whom the sinus is still patent, radiosurgery should be the primary surgical procedure. In patients in whom the tumor is larger, they recommend planned, second-stage radiosurgery. In 2006, we reported our experience with gamma knife radiosurgery in 43 patients with parasagittal meningiomas that invaded the SSS.8 Twenty-eight patients had undergone previous resection, and the follow-up period after radiosurgery ranged from 24 to 86 months (median 46 months). The median dose was 15 Gy. During this follow-up, 22 tumors (51%) decreased in size, 16 (37%) remained volumetrically unchanged, and five (12%) grew. The overall rate of tumor control with radiosurgery was 88%. Based on a minimum of 2 years’ follow-up, our results show that radiosurgery provided successful tumor control in 13 of the 17 patients with recurrent meningiomas and 10 of the 11 patients with residual tumor tissue. The discrepancy relates to small tumor volumes in residual cases, resulting in possibly higher marginal doses. Our data also indicated that, at 2 years’ follow-up, gamma knife radiosurgery controlled tumor growth 100% in virgin cases. We found that radiosurgery was ineffective in two patients with malignant meningiomas. We therefore do not recommend radiosurgery as the first-line treatment for these cases. The overall recurrence rate for the 24 residual or recurrent meningiomas with a typical histology was 8%, a rate comparable to the recurrence rates reported after gross total resection. Our surgical strategy for these tumors in the earlier years was total excision with removal of the affected SSS wall and grafting for repair. However, in 1997 we changed our policy. We now remove the gross mass of the tumor, coagulate the infiltrated portion of the sinus wall, and then assess with MRI within the first 24 hours. Our management of parasagittal meningiomas is currently based on the following considerations: 1. Parasagittal and falx meningiomas invading the SSS are common, and frequently present with neurologic symptomatology. Most of these tumors grow at follow-up; therefore, treatment is indicated. 2. The general consensus for managing tumors in the anterior third is surgery regardless of sinus invasion. Radiosurgery is also an option for patients with small meningiomas. 3. Conservative resection is associated with a high rate of regrowth. The increased morbidity rates with each repeat surgery speak against this treatment option. 4. Radical surgical resection combined with sinus reconstruction is associated with significant morbidity, low efficiency, and similarly high recurrence rates. 5. Radiation treatment is associated with significant morbidity and is reserved as a salvage treatment in recurrent cases unresponsive to other treatment methods. 6. Radiosurgery is an exciting treatment strategy and can be used as a primary treatment in all patients with small parasagittal meningiomas, unless there is a significant mass effect or peritumoral edema. Radiosurgery can also be effectively administered as an adjuvant therapy after maximal surgical resection outside the sinus to decrease the risk of regrowth. Short- and medium-term results indicate that this is a safe and effective strategy. Long-term results are yet to be determined. The presented case illustrates the difficulties of surgical decision making. The T1-weighted MRI shows that the tumor is located in the posterior part of the SSS, in the middle third, and invades at least the ipsilateral wall of the sinus, and perhaps also its roof. From these images and the venous MR angiogram, it seems impossible to ascertain whether the sinus lumen is totally or only subtotally occluded. The venous collateral circulation visible on the oblique image of the venous MR angiogram might be, in part, a still open (or recanalized) sinus lumen or, in part, satellite channels belonging to the afferent cortical veins. In addition, the picture shows (emissary) intradiploic drainage for the anterior third of the SSS. These paths of drainage contribute to the collateral circulation of the partially (or totally) occluded sinus. For this otherwise healthy young woman, two surgical approaches are possible: gross tumor removal with coagulation of the invaded sinus walls leaving an intrasinusal fragment in place, or radical removal with or without restoration of the venous circulation. Our preference would be to first remove the extrasinusal portion of the meningioma after debulking the tumor, with dissection of its so-called capsula from the brain cortex. We would then resect the invaded walls and restore circulation with a patch. To reduce the risk of recurrence of a parasagittal meningioma, our current attitude is to attempt whenever possible a total removal of the tumor, including the invaded walls or the occluded portion of the sinus. Such a decision to radically remove the tumor leads to an aggressive surgical approach, which implies restoration of the venous circulation. In the “dangerous” case presented here, our decision to radically remove the tumor and restore the venous circulation is reinforced by the tumor’s location within the province of rolandic outflow. Impairment in this location would probably cause hemorrhagic infarction in crucial sensori-motor territories. Because the meningioma in this patient likely does not invade the contralateral wall, it would be possible to restore flow with a patch. Fig. 2.1 The authors’ classification of parasagittal meningiomas.11,12,15,33 Type I: The meningioma is attached to the outer layer of the sinus wall. Type II: The lateral recess is invaded. Type III: The ipsilateral wall is invaded. Type IV: Both the ipsilateral wall and the roof of the sinus are invaded. Type V: The lateral wall and the roof are invaded and the lumen is occluded, but the contra-lateral wall is free of invasion. Type VI: All sinus walls are invaded and the sinus is totally occluded. Our surgical experience comprises a series of 160 meningiomas involving the major dural venous sinuses.11,12,15,33 This experience has led us to adopt the following surgical strategies based on the classification of these meningiomas into six types,11,12,15,27,33 as shown in Fig. 2.1: Type I: The meningioma is attached to the sole outer layer of the sinus wall. The outer dural layer is resected and the site of attachment coagulated, leaving a clean and glistening dural surface. Type II: The lateral recess is invaded. The intrasinusal fragment is extracted and the dural defect repaired with either a simple running suture or a patch made of autologous aponeurosis (dura mater, periosteum, fascia lata, or, better, fascia temporalis). Type III: The ipsilateral lateral wall is invaded. The invaded wall is resected and repaired with a patch. Type IV: The ipsilateral wall and the roof are both invaded. The invaded walls are resected and the sinus is kept patent with a patch reconstruction. Types V and VI: The sinus is totally occluded. In Type V, one wall is free of tumor. Although the current theory is to remove the invaded portion of the sinus without reestablishing venous circulation, our preference is to restore this circulation. In tumors of type V, circulation can be restored with a patch after the two invaded walls (or ipsilateral wall and roof) have been resected and the fragment that occludes the sinus extracted (Fig. 2.2). In tumors of type VI, restoration must be done with a bypass, which can be end-to-side before tumor removal or end-to-end after removal. For meningiomas in the posterior third of the SSS, the torcular or the lateral sinus, a sino-jugular bypass at the neck can be done before sacrifice of the invaded portion (Fig. 2.3).
Management of Parasagittal Meningiomas Involving the Superior Sagittal Sinus: Partial Removal with Radiosurgery vs. Total Removal with Repair
Combined Surgical and Radiosurgical Treatment of Parasagittal and Falx Meningiomas with Superior Sagittal Sinus Invasion
General Considerations
Management Strategy
The Role of Radiosurgery
Conclusion
Total Removal of Parasagittal Meningiomas Involving the Superior Sagittal Sinus with Sinus Repair
Case Discussion
General Considerations
Classification
Management of Parasagittal Meningiomas Involving the Superior Sagittal Sinus: Partial Removal with Radiosurgery vs. Total Removal with Repair
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree