44 Management of Skull Base Defects After Extended Endoscopic Skull Base Surgery: From Free Grafts to Vascularized Flaps
Gustavo Hadad, Luis Bassagaisteguy, Daniel Timperley, and Aldo Cassol Stamm
 Introduction
Introduction
During the 1980s a patient who presented with a tumor of the anterior skull base was offered open craniofacial surgery and skull base reconstruction with galea and fixation with metal plates or bone flaps. Moreover, the patient would undergo a prolonged stay in an intensive care unit (ICU) with a lumbar drainage catheter and ran a high risk of infections and other types of postoperative complications.1
Twenty years later this patient has the option of minimally invasive endoscopic surgery, with maximal tumor resection by a natural orifice. This is due to the development of endoscopic transnasal craniectomy surgery.
This endoscopic access allows a wide resection of the tumor and a short hospital stay, without external incisions or lumbar drainage. However, morbidity and mortality rates are still ranging from 10 to 40%, mostly because of the inability to deal with large defects created in the skull base.2
In an effort to achieve better results, many techniques have been designed to try to reduce these complications, mainly postoperative cerebrospinal fluid (CSF) fistula and its potential infective complications such as meningitis and ventriculitis.
Several techniques have been described, including the use of free grafts and fascia lata or temporalis in an underlay or overlay fashion, with double or triple layers and use of biologic substitutes, among others.3–7 All these grafts were nonvascularized and the results were variable, depending on whether the graft gained sufficient vascular supply from the adjacent tissues.
The results were worse if there was previous irradiation at the surgical field, and multiple closure surgeries were common, further increasing the risk of infective complications.
 Nasoseptal (Hadad–Bassagaisteguy) Flap
Nasoseptal (Hadad–Bassagaisteguy) Flap
History
In 1996, Drs. Gustavo Hadad and Luis Bassagaisteguy, from Argentina, developed their first nasal septal mucoperiosteal flap in the form of a sliding rotation flap that could not cover all skull base defects. Based on the concept of regional flaps in the reconstruction of other parts of the body, these nasal septal flaps also resulted in improved closure and, because they were vascularized, less retraction than with traditional free grafts.
The flaps were harvested at the end of the tumor removal procedure. The relatively simple technique kept a wide pedicle to ensure vascular supply. However, the wide pedicle restricted the range of rotation, limiting the ability to position the flap due to the risk of compromising the vascular supply.
After cadaver studies, the doctors from Argentina designed an axial flap with a narrow pedicle, based on a single artery, which allowed rotation in any direction while protecting the artery and maintaining vascular supply.
The sphenopalatine artery was the vessel with the largest possible vascular territory. Because of the course of its septal branch arching over the posterior choana into the nasal septum, it allowed the creation of large vascularized flaps with a wide arc of rotation.
The Hadad–Bassagaisteguy flap (HBF), pedicled on the sphenopalatine artery, was finally developed in 1999, and, shortly after, the first clinical human studies were successfully performed in patients with iatrogenic CSF leaks after endoscopic sinus surgery.
The generous dimensions of the flap, its versatility, and the ease of design with a robust vascular pedicle enabled surgeons to perform the first procedure in 1999 using the HBF in the closure of a large skull base CSF fistula, after craniofacial surgery for the resection of a meningoencephalocele.
Since 2005, the HBF has been consistently used for skull base closure at the University of Pittsburgh, the Sao Paulo ENT Center, and many other institutions through the world. The short- and long-term complications of CSF fistula, infections, and postoperative morbidity and mortality have decreased dramatically with the use of the HBF.8–10
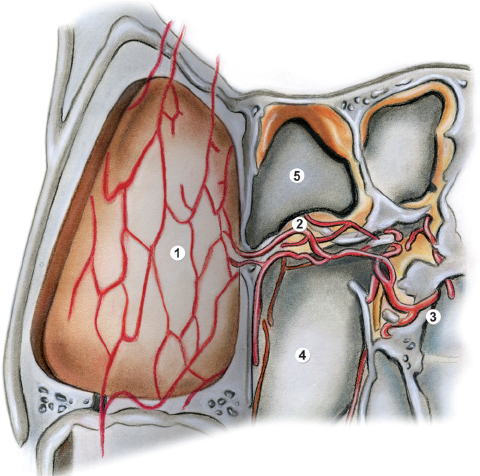
Fig. 44.1 Drawing showing the vascular supply of the Hadad– Bassagaisteguy flap (HBF). 1, donor site for raising the flap from the septal mucosa; 2, area of the nasoseptal artery; 3, sphenopalatine artery; 4, left posterior choana; 5, sphenoid sinus.
Anatomy and Surgical Technique
The sphenopalatine artery is the terminal branch of the internal maxillary artery, and it enters the nose through the sphenopalatine foramen after traversing the pterygopalatine fossa. At the foramen it then divides into two branches.
The anterior branch runs along the inferior turbinate and nasal lateral wall. The posterior branch (septal artery) runs over the rim of the posterior choana on the sphenoid bone to the apex of the vomer, where it divides into a network of arteries running through the septum from posterior to anterior in the submucosal plane above the periosteum and perichondrium.
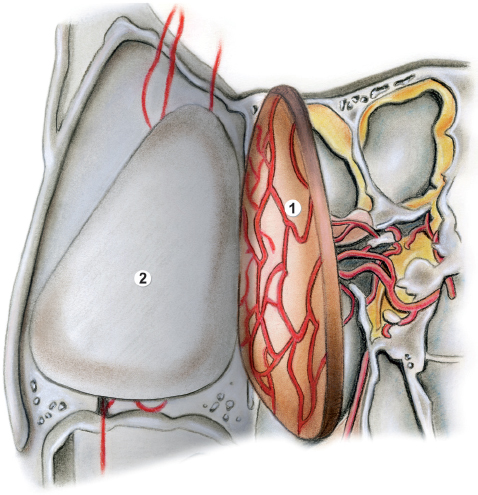
Fig. 44.2 Drawing of the anatomy for the surgical procedure for the HBC (continued). 1, nasoseptal flap separated from the nasal cartilage; 2, denuded septal cartilage.
This fact allows the flap to be harvested from the nasal septum in a subperichondrial and subperiosteal fashion (Figs. 44.1, 44.2, and 44.3). The creation of the flap starts at the beginning of the surgery. It is limited posteriorly by the posterior choana. Following topical vasoconstriction and local infiltration with adrenalin containing local anesthetic, the middle and superior turbinates are lateralized or resected to expose the nasal septum from cribriform to nasal floor. An inferior horizontal incision is made 0.5 cm above the nasal floor. The anterior limit is at the entrance of the nostril. It is important to remember that this will determine the length of the HBF. If the flap is intended to close a skull base defect of the anterior cribriform, it should be designed as long as possible, because it is pedicled posteriorly. Posteriorly, this incision follows the posterior border of the nasal septum and then arches over the posterior choana just below the front face of the sphenoid. The superior horizontal incision is usually performed 1 cm below the cribriform. Posteriorly this incision crosses the sphenoid face at the level of the inferior border of the natural sphenoid ostium. A vertical incision joins the two horizontal incisions anteriorly. The anterior reach of the flap can be improved if required by extending the horizontal incisions onto the lateral nasal wall. The inferior incision is continued forward at the level of the inferior turbinate insertion, and the superior incision is made above the level of the sphenopalatine foramen. The flap can then be elevated as far forward as the sphenopalatine foramen. If necessary, the anterior aspect of the foramen and part of the posterior wall of the maxillary sinus are opened using Kerrison rongeurs, and the vessels are dissected free to further improve the reach of the flap.
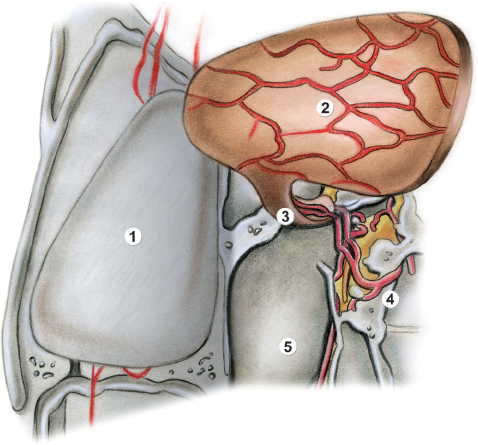
Fig. 44.3 Drawing of the anatomy for the surgical procedure for the HBF (continued). 1, denuded septal cartilage; 2, elevated nasoseptal flap; 3, mucosa and vascular pedicle through which the flap receives blood supply by the nasoseptal artery; 4, sphenopalatine artery; 5, left posterior choana.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



