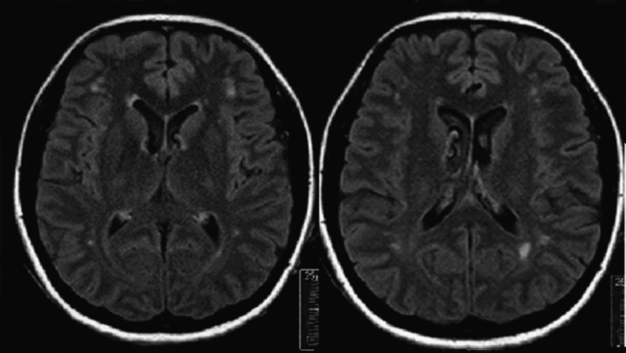
Case 1. Brain MRI FLAIR (a) and T2 sequences (b) showing punctate subcortical white matter lesions. None were located on the cerebellum or in the cerebellar or vestibular pathways.
On examination pulse was regular. Sitting blood pressure (BP) was 105/70 mmHg, with no orthostatic changes. General physical and neurologic examinations were unremarkable; examination of the V, VII, and VIII cranial nerves was normal and there were no vestibular or cerebellar signs. There was no base widening or side deviations while walking, but the patient was cautious and felt uncomfortable walking with eyes closed. The Dix–Hallpike maneuver with the head rotated to the right side produced intense vertigo, nausea, and sweating, reproducing the symptoms the patient reported during her spells. Vertigo started a few seconds after the patient lay down, lasted for about 20 seconds, and was accompanied by a rotational geotropic nystagmus, as the top pole of the eyes rotated towards the undermost ear. A similar, although less intense, vertigo was noticed when the patient returned to the seating position. Vertigo became less intense and of shorter duration, with repetitive testing.
Canalith repositioning was successfully attempted using the Epley maneuver. The patient was instructed not to sleep completely flat for 2 days, to use dimenhydrinate 50 mg if too nauseated, and to start a home program of vestibular rehabilitation exercises. She was strongly advised to quit smoking.
Discussion
This active healthy woman complained of multiple daily episodes of vertigo and gait unsteadiness for a period of 4 weeks. She was diagnosed as having multiple transient ischemic attacks (TIAs). MRI was ordered and spotty subcortical white matter changes were read as “small strokes.” There are several arguments making the diagnosis of TIA implausible in this case: (1) the patient is a young adult and has just one vascular risk factor, she was not diabetic and her blood pressure was normal; (2) the temporal pattern of the episodes does not suggest a vascular cause, as there were multiple daily episodes for a period of 4 weeks. Multiple TIAs can occur, but in general are spaced by days or weeks. A clustered pattern (cluster or crescendo TIA) is also possible in the capsular and pontine warning syndromes [7–9], showing repetitive episodes of hemiplegia, but only for a few days; (3) the spells consisted only of vertigo and the corresponding unsteadiness, plus profuse vegetative symptoms, which point to a peripheral, vestibular origin of the symptoms. Diplopia, numbness, dysphagia, dysarthria, hiccups, or other intra-axial symptoms indicating brainstem ischemia were not reported. Restricted cerebellar infarcts presented isolated vertigo [10], with abnormal vestibular signs on neurological examination. In this case neurological examination was normal between the episodes. Isolated transient episodes of vertigo should not be considered as due to transient brainstem ischemia, unless accompanied by other brainstem, cerebellar, or occipital symptomatology [11].
Neurologic examination between episodes was normal. However, the Dix–Hallpike maneuver replicated the symptoms experienced by the patient and provided evidence in favor of a peripheral vestibular disorder. In fact, vertigo and nystagmus appeared only when the head was rotated to the right side, there was a latency of seconds before their onset, they faded away spontaneously, and their intensity decreased as the maneuver was repeated, i.e., nystagmus was fatigable. This observation, taken together with the patient’s complaints, is sufficient to establish the diagnosis of benign paroxysmal positional vertigo (BPPV) caused by cupololithiasis of the posterior semicircular canal. As its name indicates, BPPV is a benign and frequent condition, which causes recurrent attacks of positional vertigo. A repositioning maneuver is the recommended treatment in the acute phase [12].
MRI showed a few punctate subcortical white matter lesions (WML). Diffusion-weighted imaging (DWI) was negative. WML were interpreted as lacunar infarcts. None were located in the cerebellum or in the vestibular nuclei or pathways. Therefore, WML could not be responsible for the patient’s symptomatology. These punctate lesions are smaller than lacunar infarcts and correspond to areas of gliosis. They represent a perivascular reduction in myelin content with atrophy of the neuropil and seem to constitute a negligible extent of tissue damage from low permeability of thickened arteriolar walls [13]. Their frequency increases with age, especially after 60, but they are often detected in MRIs of younger adults, in particular if they have vascular risk factors or migraine. Large cohort studies clearly showed that these punctate lesions (grade 1 in Fazekas’ scale) do not increase with time, in contrast to the progression to more extensive lesions seen in early confluent (grade 2) or confluent (grade 3) WML [14,15]. Antiplatelets are not recommended for patients with WML without stroke or TIA. There is no evidence that quitting smoking prevents progression of WML, but quitting smoking definitively reduces the risk of further vascular events including stroke. Any medical encounter is an opportunity to advise smokers to quit their “suicidal” habit.
Tip
Recurrent attacks of intense vertigo with nausea triggered by head or body movement to one side were rather suggestive of BPPV. The characteristic response on the Dix–Hallpike maneuver confirmed the diagnosis.
Pitfall
The ENT specialist failed to perform the appropriate physical examination of a patient with vertigo, which should include the Dix–Hallpike maneuver. Instead he asked for an unnecessary MRI. He misinterpreted benign minor white matter changes as lacunar stroke. He also did not perform a clinical topographical diagnosis, as none of the visible white matter changes were located in a brain area whose damage would produce vertigo.
Case 2. Iatrogenic CT
Case report
A 46-year-old secretary was referred to the neurology outpatient clinic because of “silent strokes.” She had no known vascular risk factors, although she did not check her BP. Her past medical history was unremarkable, except for a long-standing history of tension headache, without analgesic abuse, and recent complaints of job-related anxiety and dry mouth. She had no episodes of sudden focal neurologic deficits, suggesting TIAs or stroke. Brain imaging was performed at another institution. Brain CT raised question of a sphenoid ridge meningioma and therefore an MRI was ordered. MRI ruled out meningioma, but showed linear T2 hyperintense periventricular white matter lesions and a few frontal punctate subcortical white matter lesions (Figure 18.2). DWI was normal. The resident who first examined the patient found no abnormalities on the neurologic examination, but recorded a sitting BP of 130/85 mmHg. He ordered blood tests, including autoimmunity panel and Borrelia serology, extra-and intracranial vascular ultrasound, cervical MR, and visual evoked potentials. All these tests gave negative or normal results. An ambulatory 24-hour BP recording was later recorded, which showed that the patient was a non-“dipper.” She was started on a beta-blocker.
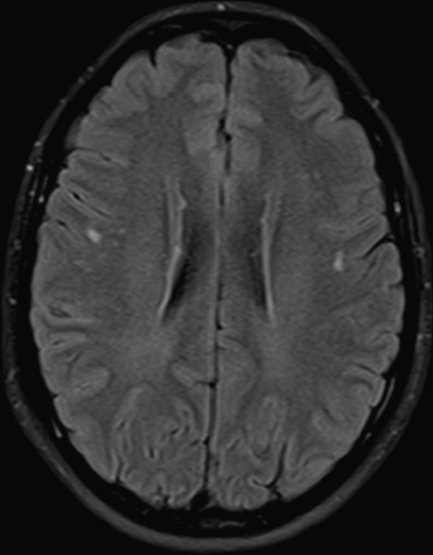
Case 2. Brain MRI FLAIR depicting periventricular and punctate subcortical white matter lesions.
Discussion
As in the previous case, MRI showed white matter changes, both periventricular and in the deep white matter. Periventricular white matter lesions can be shown in T2 and Fluid Attenuated Inversion Recovery (FLAIR) sequences at the ventral and dorsal limits of the lateral ventricles and have the shape of a cap. They can also appear as a smooth linear halo on the lateral margins of the lateral ventricles. Periventricular caps are characterized pathologically by loosely arranged fine-fiber tracts with low myelin and high extracellular fluid content. Patchy loss of the ependyma with astrocytic gliosis is frequently observed. Smooth periventricular halo has been linked to the disruption of the ependymal lining with subependymal gliosis and subsequent loss of myelin. It has also been found to be related to venous congestion due to noninflammatory periventricular venous collagenosis [13,16,17]. This means that these lesions can hardly be considered of ischemic vascular origin.
MR findings were unrelated to the tension headaches which brought the patient to the consultation. This incidental finding provoked the request of a series of other tests which fortunately were all normal. Otherwise, other or confirmatory tests would be performed, increasing exponentially the risk of new incidental findings. The cost and worries for the patient were not negligible. All tests were ordered to exclude remote possible diagnoses, not taking into consideration the very low pre-test probability of a positive result. As vascular white matter lesions are a manifestation of small vessel disease, ultrasound or angiographic evaluation of the extracranial vessels usually gives normal results or shows minimal stenosis or plaques. The presence and intensity of white matter changes does not have any relationship to the degree of carotid stenosis eventually found in carotid ultrasound [18]. In other words, performing carotid ultrasound in patients with WML has a yield similar to performing it in “normal” individuals. Considering laboratory tests, Lyme disease should not be considered in the differential diagnosis of hyperintense T2 foci in middle-aged and elderly patients, except if clinically suspected [19]. Dry mouth might raise the possibility of Sjögren’s syndrome with cerebral involvement, although other features (dry eye, arthralgias) were absent and dry mouth could be interpreted in the context of anxious symptomatology. WML are not more frequent in Sjögren’s syndrome than in age-matched controls. Even in Sjögren’s syndrome WML are associated with increasing age, diabetes, and hypertension [20]. Several community- and hospital-based studies have established that increasing age, diabetes, and hypertension are the most important risk factors for the appearance of WML. Fasting or casual glycaemia and hemoglobin A1c were performed to detect diabetes. Measurement of blood pressure during outpatient consultation showed borderline values and she would be classified as prehypertensive. Ambulatory 24-hour monitoring of blood pressure revealed that she was a non-dipper, as her BP failed to decrease during sleep. After this result she was started on an antihypertensive, to decrease her risk of vascular events, mainly of stroke. Subcortical white matter lesions are a marker of hypertensive end-organ damage. Its presence has been related to casual or 24-hour BP values, to non-dipper and inverted dipper patterns, to blood pressure surge in the morning, and to blood pressure variability [21].
Tip
Discrete punctate age-related WML do not need extensive ancillary investigation looking for vasculitis and demyelinating disorders. Ambulatory 24-hour BP monitoring is more sensitive than casual office BP measurement to detect hypertension.
Pitfall
WML of vascular origin are associated with small vessel disease. Ultrasound or angiography of the carotids and vertebral arteries, searching for a significantly hemodynamic stenosis, is only recommended when the lesions have a watershed spatial distribution pattern, i.e., when they are located between two cerebral arterial territories.
Case 3. A painful experience
Case report
A 46-year-old nurse, with a previous history of migraines without aura since adolescence and treated hypertension, came to the emergency room (ER) because of aphasia of sudden onset, which had started half an hour before. She suddenly experienced difficulty in recalling the names of common objects and she produced a few made-up words. She was fully aware of the defect and had no trouble understanding language. Examination confirmed anomia in visual confrontation naming with the production of some neologistic paraphasias. No other abnormalities were found. The pulse was regular, sitting BP was 135/80 mmHg and no carotid or cardiac murmurs were heard. While in the ER, she started experiencing a severe throbbing right-sided temporo-orbital headache, with photophobia and sonophobia. She felt nauseated. The headache was similar to her usual migraine. Two hours later the speech defect started to improve, but it was followed by a binocular disturbed vision, which she described as scintillating “white squares.” One hour later, all her symptoms had gone. A brain CT did not reveal any early infarct changes or any other lesion except for some diffuse subcortical white matter hypodensities. A brain MRI was then ordered. DWI did not show any area of restricted diffusion. MRI confirmed the presence of scattered T2 and FLAIR hyperintense images in the frontal and subcortical white matter (Figure 18.3). There were no such lesions either on the temporal lobe or on the occipital lobe. The resident on duty admitted the patient for further evaluation, with the possible diagnosis of vasculitis. All ancillary procedures performed were normal, including angio-MR, cervical vascular ultrasound, echocardiography, Holter, blood tests (hematology, biochemistry, syphilis, HIV, hepatitis and Borrelia serologies, and prothrombotic and immunological screening panels). Lumbar puncture (LP) revealed a normal cerebrospinal fluid with no increased cell number or oligoclonal bands. Spine MRI and multimodal evoked potential were also normal. The patient was discharged on her previous antihypertensive medication with a diagnosis of migraine with aura.
Discussion
This hypertensive middle-aged nurse had an episode of migraine with aura with some less usual features, which increased the uncertainty about the diagnosis of her current event. Uncertainty leads to a series of unnecessary, costly, and painful experiences including hospital admission and LP. Less common features of the migraine attack included first episode of aura after a long history of migraine without aura, and aura which had an apparently sudden onset and was somewhat prolonged. It is not uncommon that a migraineur who has always had only migraine without aura experiences by his forties–fifties a few or a series of migraine auras, often without headache. These episodes often raise the suspicion of TIA/ischemic stroke. Auras usually have a progressive onset and last a median of half an hour. These are the central values of a biological normal distribution. The extremes of such a distribution are the cases with rapid onset or lasting a few hours. Otherwise this nurse’s headache was typical of migraine and was similar in quality and intensity to her previous attacks [22]. The aura, although prolonged, had a typical progression. In fact, as the speech defect cleared, a transient binocular scintillating scotoma appeared.
In 1980, Miller-Fisher presented 120 cases with what he coined late-life migrainous accompaniments resembling TIAs [23]. In 1986, 85 further cases examined over 5 years were described, supporting the concept advanced previously [24]. In general, the cases had auras with a variable combination of visual disturbances, paresthesias, or speech disturbance. He stressed that “The ages ranged from 40 to 73 years. Headache occurred in association with the episodes in only 40% of cases” and concluded that “The condition can justifiably be regarded as benign. Migrainous accompaniments account for some of the cases of transient ischemia with normal angiograms. Knowledge of the condition helps in the planning of rational management.” The current case confirms the accuracy of his detailed observations and judicious remarks.
As MRIs started to be performed in patients with migraine for a variety of reasons (often unnecessary…), it became evident that WMLs were frequent in these patients. A meta-analysis showed that the WML were 3.9 times more frequent in people with migraine [25]. Some studies found an association with duration of migraine history and with the frequency of attacks. Right-to-left shunt does not increase this lesion load. In the ARIC study migraine was associated with white matter hyperintensity (WMH) volume cross-sectionally but not with WMH progression over time [26]. This suggests that the association between migraine and WMH is stable in older age and may be primarily attributable to changes occurring earlier in life. Therefore antiplatelet drugs should not be prescribed to migraineurs because of WMLs mislabeled as “small strokes.”
Tip
Subcortical white matter changes are very common in migraineurs. These white matter changes do not represent strokes, vasculitis, or demyelinating disorders and do not warrant any ancillary investigation.
Case 4. Stroke in an army officer, with multiple causes
Case report
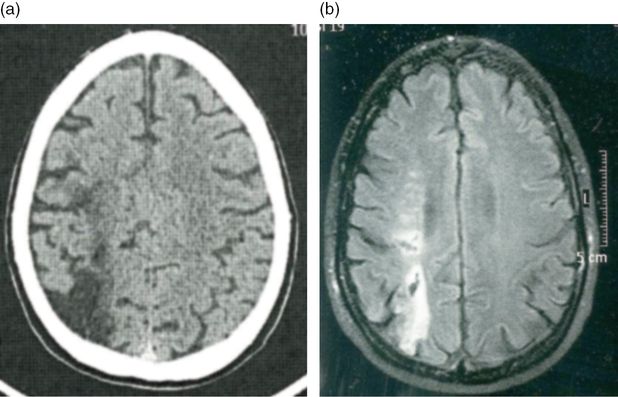
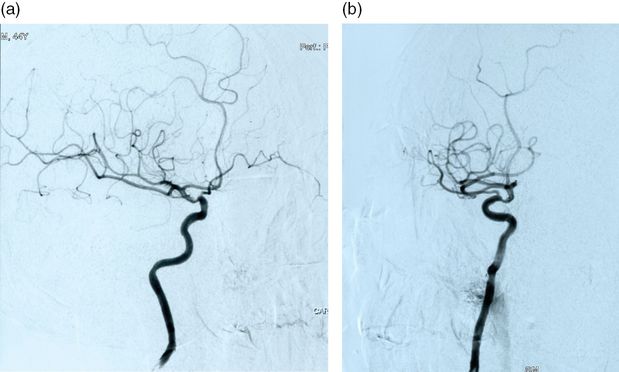
A 45-year-old army officer noticed left-sided hand weakness and blurred vision on his left side on awakening. He was a smoker of 15 cigarettes a day but had no other risk factors. He had been on a military training mission in Angola a month before. He denied head or cervical trauma. When seen at the clinic a month later, neurologic examination showed decreased position sense and tactile hypoesthesia in his right hand with pseudoathetotic posturing of the right fingers. A discharge note from another hospital described a left lower quadrantanopsia, which was no longer detected. A right parietal infarct was apparent on brain CT. CT angiography was also performed and no occlusion or stenosis was identified (Figure 18.4). Additionally, two small internal carotid artery aneurysms, one on each carotid, measuring on the right carotid 2.8 mm and 3.3 mm on the left carotid, were reported. Blood analysis were normal except for a cholesterol level of 221 mg/dL and positive anticardiolipin IgG (41 GPLU/mL) and antiβ-2 glycoprotein (34 UA/mL). Hepatitis, syphilis, and HIV serologies were negative. Chest X-ray, ECG, and transthoracic and transesophageal echocardiogram were unremarkable. He was discharged on aspirin and atorvastatin. We ordered a brain MRI, which showed two right hemispheric ischemic infarcts with hemorrhagic transformation, a parietal one already visible on CT and a second one located in the parieto-frontal white matter (Figure 18.5). These infarcts were located in the “watershed” areas between the middle cerebral, anterior cerebral, and posterior cerebral artery territories. MR angiography showed decreased diameter and slow flow on the petro-cavernous segment of the right internal carotid and failed to detect the aneurysms, previously shown on CT angiography (Figure 18.6). Repeated testing for antiphospholipid antibodies showed very high titers for lupus anticoagulant, anticardiolipin (104 UGPL/mL), and antiβ-2 glycoprotein (54 UA/mL). Immunological screening for vasculitis and lupus was negative. Because of the twice positive results for antiphospholipid antibodies, the patient was started on warfarin. Considering the probable need for prolonged anticoagulation in a patient harboring two unruptured aneurysms, and the contradictory results of CT and MR angiographies, an intra-arterial angiography was requested. No aneurysms were found but a stenosis of the supraclinoid segment of the right carotid artery was apparent (Figure 18.7). The patient stopped smoking, warfarin was continued, and non-invasive follow-up of the carotid stenosis was planned.
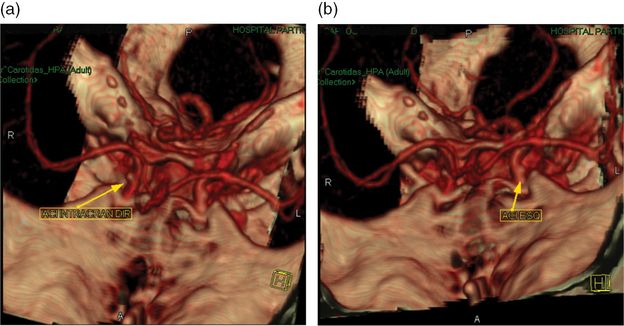
Case 4. CT angiography, showing small “aneurysms” in both intracranial carotid arteries.
Discussion
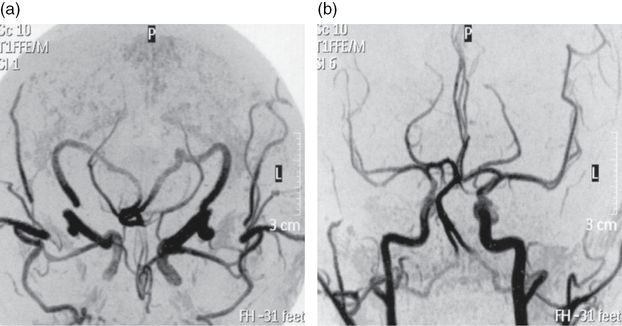
This young, physically active smoker suffered a right hemispheric stroke on awakening. The CT and MRI territorial distribution of the infarct was located in the boundaries of the middle cerebral, posterior cerebral, and anterior cerebral artery territories. These “watershed infarcts” have usually a hemodynamic mechanism, due to a severe stenosis or occlusion of a proximal artery, in general the ipsilateral internal carotid. Less often “watershed” infarcts are caused by microembolism. CT angiogram failed to identify any extra or intracranial arterial stenosis or occlusion. Also no cardiac or aortic cause of embolism was detected. Two images, read as small unruptured carotid aneurysms, were incidentally discovered by CT angiography. On the other hand the pursuit of an etiology for the stroke led to the diagnosis of antiphospholipid syndrome, based on high titers of antiphospholipid antibodies on two different measurements. Antiphospholipid syndrome carries a high risk of recurrent thrombotic events and prolonged oral anticoagulation is recommended to decrease such risk. Anticoagulation was started, but further investigation was carried on, exploring the possibility of treatment of the carotid aneurysms, despite their small size, considering the need for prolonged anticoagulation. Neither angio-MR nor intra-arterial angiography confirmed the “aneurysms” seen on CT angiograms, which were probably false images produced by vessel overlap or angulation. On the other hand angio-MR raised the suspicion of a stenosis of the petro-cavernous segment of the internal carotid, which was confirmed by intra-arterial angiography. The nature of the intracranial stenosis cannot be defined beyond doubt. Partially recanalized dissection, congenital stenosis, and intracranial atheroma are etiologic possibilities. The first two entities have a very low risk of progression or embolism. Intracranial carotid stenosis has a high risk of recurrent stroke. Neither warfarin nor endovascular treatment do better than a single antiplatelet regimen to decrease such risk [27]. Warfarin was continued because of the antiphospholipid syndrome [28].
CT and MR angiography have replaced intra-arterial angiography in the evaluation of ischemic stroke, moving it to a second-line diagnostic procedure for doubtful cases or when endovascular or revascularization neurosurgery is planned. However, both CT and MR angiography have a lower precision than intra-arterial angiography [29–31], and can produce either false negative (no intracranial stenosis) or false positive (aneurysms) diagnoses, as this case exemplifies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree