17 Median Neuropathy at the Wrist
Anatomy
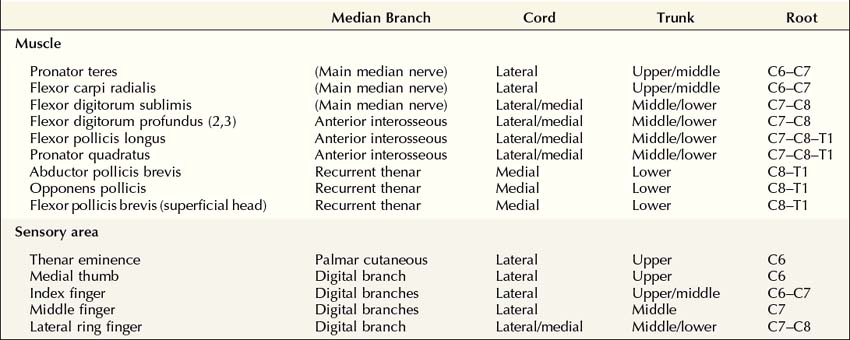
Understanding the anatomy of the median nerve is the first step toward being able to differentiate entrapment of the median nerve at the wrist from lesions of the proximal median nerve, brachial plexus, and cervical nerve roots, on both clinical and electrophysiologic grounds. The median nerve is formed by a combination of the lateral and medial cords of the brachial plexus (Table 17–1, Figure 17–1). The lateral cord is made up of C6–C7 fibers and supplies median sensory fibers to the thenar eminence, thumb, index, and middle fingers, and motor fibers to the proximal median forearm muscles. The medial cord, composed of C8–T1 fibers, supplies motor fibers to the median muscles of the distal forearm and hand, as well as sensory fibers to the lateral half of the ring finger.

FIGURE 17–1 Anatomy of the median nerve.
(Adapted with permission from Haymaker, W., Woodhall, B., 1953. Peripheral nerve injuries. WB Saunders, Philadelphia.)
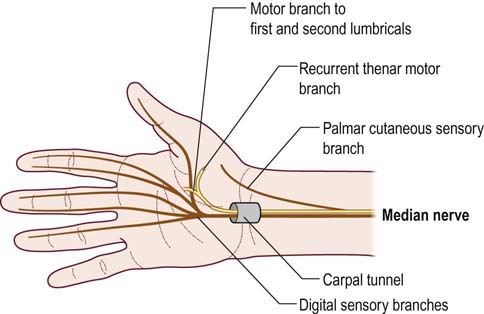
Just proximal to the wrist and carpal tunnel, the palmar cutaneous sensory branch arises next, running subcutaneously to supply sensation over the thenar eminence. The median nerve then enters the wrist through the carpal tunnel. Carpal bones make up the floor and sides of the carpal tunnel, and the thick transverse carpal ligament forms the roof (Figure 17–2). In addition to the median nerve, nine flexor tendons traverse the carpal tunnel as well (FDP: four tendons; FDS: four tendons; FPL: one tendon). In the palm, the median nerve divides into motor and sensory divisions. The motor division travels distally into the palm, supplying the first and second lumbricals (1L, 2L). In addition, the recurrent thenar motor branch is given off. This branch turns around (hence, recurrent) to supply muscular branches to most of the thenar eminence, including the opponens pollicis (OP), abductor pollicis brevis (APB), and superficial head of the flexor pollicis brevis (FPB). The sensory fibers of the median nerve that course though the carpal tunnel supply the medial thumb, index finger, middle finger, and lateral half of the ring finger. The index and middle fingers are each supplied by two digital branches (one lateral and one medial); the thumb and ring fingers receive only one branch each (Figure 17–3).
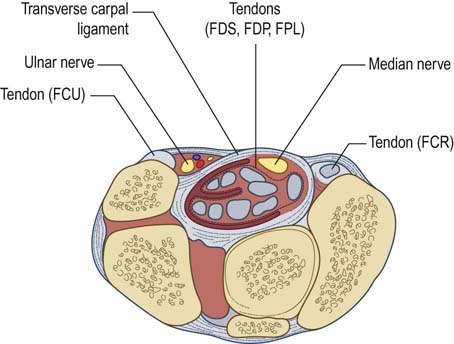
FIGURE 17–2 Anatomy of the median nerve at the carpal tunnel.
(Reprinted with permission from Pecina, M.M., Krmpotic, Nemanic, J., Markiewitz, A.D., 1991. Tunnel syndromes. CRC Press, Boca Raton, FL.)
Clinical
Patients with CTS may present with a variety of symptoms and signs (Table 17–2). Women are affected more often than men. Although CTS usually is bilateral both clinically and electrically, the dominant hand usually is more severely affected, especially in idiopathic cases. Patients complain of wrist and arm pain associated with paresthesias in the hand. The pain may be localized to the wrist or may radiate to the forearm, arm, or, rarely, the shoulder; the neck is not affected. Some patients may describe a diffuse, poorly localized ache involving the entire arm. Paresthesias are frequently present in the median nerve distribution (medial thumb, index, middle, and lateral ring fingers). Although many patients report that the entire hand falls asleep, if asked directly about little finger involvement, most will subsequently note that the little finger is spared.
Table 17–2 Clinical Symptoms and Signs
| Highly Suggestive of Carpal Tunnel Syndrome | Possible Carpal Tunnel Syndrome | Inconsistent with Carpal Tunnel Syndrome |
|---|---|---|
| Nocturnal paresthesias awakening patient from sleep | Hand, wrist, forearm, arm, and/or shoulder pain | Neck pain |
| Shaking or ringing the hands | ||
| Pain/paresthesias associated with driving or holding a phone, book, or newspaper | Perception of paresthesias involving all five digits | Paresthesias radiating from neck and shoulder down the arm |
| Sensory disturbance of digits 1,2, 3, and 4, splitting the fourth digit | No fixed sensory disturbance, or sensory disturbance of digits 1, 2, 3, and/or 4 | Unequivocal numbness over the thenar eminence |
| Weakness/wasting of thenar eminence | Decreased hand dexterity | Weakness/wasting of hypothenar muscles, thumb flexion (interphalangeal joint), arm pronation, and/or elbow flexion/extension |
| Phalen’s maneuver reproduces symptoms | Tinel’s sign over the median nerve at the wrist | Reduced biceps or triceps reflexes |
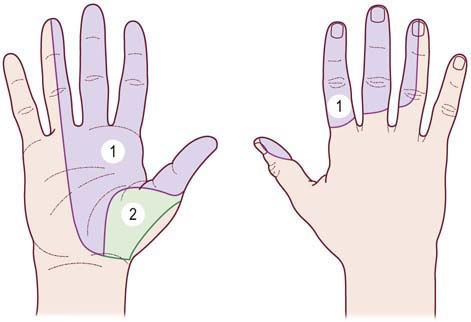
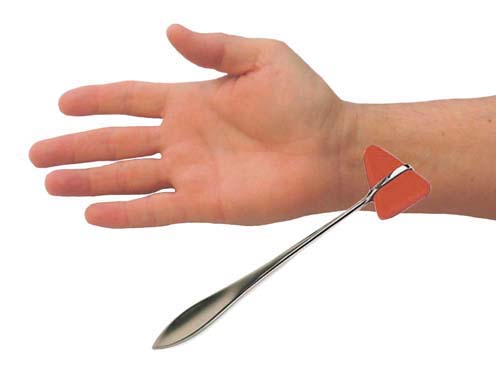
The sensory examination may disclose hypesthesia in the median distribution. Comparing sensation over the lateral ring finger (median innervated) to that over the medial ring finger (ulnar innervated) is often helpful. Sensation over the thenar area is spared because this area is innervated by the palmar cutaneous sensory branch, which arises proximal to the carpal tunnel (Figure 17–4). The Tinel’s sign is often present when tapping over the median nerve at the wrist, which results in paresthesias in the median-innervated fingers (Figure 17–5). The Phalen’s maneuver, whereby the wrist is held passively flexed, may also provoke symptoms (Figure 17–6, top). A wide range of sensitivities and specificities for the Tinel’s sign and Phalen’s maneuver have been reported in the literature. A Tinel’s sign is present in more than half of CTS cases; however, false-positive Tinel’s signs are common in the general population. A Phalen’s maneuver usually produces paresthesias within 30 seconds to 2 minutes in CTS; it is more sensitive than the Tinel’s sign and has fewer false-positive results. Most commonly, the Phalen’s maneuver will produce paresthesias in the middle or index fingers. It should be noted, however, that because the Phalen’s maneuver often is performed with the elbow flexed as well (a provocative maneuver for ulnar neuropathy at the cubital tunnel), this position occasionally may produce ulnar paresthesias in patients with ulnar neuropathy.
The motor examination involves inspection of the hand, looking for wasting of the thenar eminence (severe cases), and testing the strength of thumb abduction and opposition (Figure 17–7). Isolating the actions of the APB and OP (median-innervated muscles distal to the carpal tunnel) may be difficult because thumb abduction is also served by the abductor pollicis longus (radial nerve) and thumb opposition by a combination of the deep head of the FPB (innervated by the ulnar nerve) and the FPL (innervated by the anterior interosseous nerve).
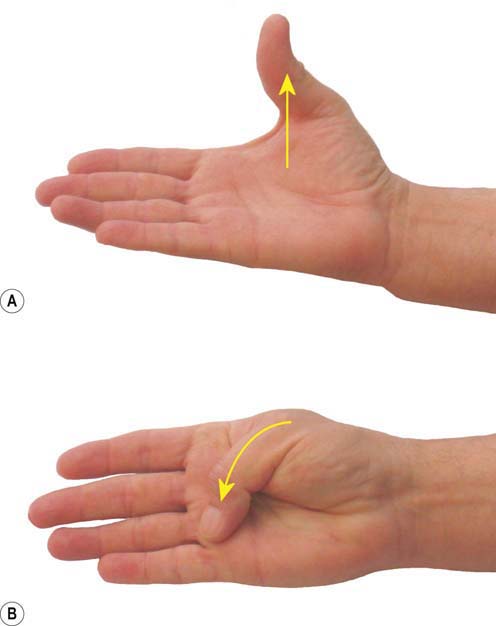
FIGURE 17–7 Muscle testing in carpal tunnel syndrome.
Thumb abduction (A) and opposition (B) may be weak in more advanced cases of carpal tunnel syndrome.
Etiology
The reported causes of CTS are numerous (Box 17–1). Despite this exhaustive list, most cases are idiopathic. Indeed, idiopathic cases present with the same signs and symptoms as CTS caused by the other conditions listed in Box 17–1. Although the etiology of idiopathic cases was long considered to be tenosynovitis of the transverse carpal ligament, pathologic evaluation typically shows little evidence of inflammation. In most cases, edema, vascular sclerosis, and fibrosis are seen, findings consistent with repeated stress to connective tissue. Compression results in symptoms by way of ischemia and demyelination and, if it is severe enough, wallerian degeneration and axonal loss.
Occupations or activities that involve repetitive hand use clearly increase the risk of CTS (e.g., typists, data entry workers, mechanics, and carpenters). From the exhaustive list given in Box 17–1, the conditions most often associated with CTS, other than idiopathic, are diabetes, hypothyroidism, rheumatoid arthritis, amyloidosis, and pregnancy. One important clue to an underlying cause, other than idiopathic, is the presence of CTS in the non-dominant hand. In idiopathic cases, the dominant hand is nearly always the affected hand; if symptoms are bilateral, then the dominant hand is more affected than the contralateral hand. CTS that is significantly worse in the non-dominant hand should raise a red flag to a specific underlying cause other than idiopathic CTS.
Electrophysiologic Evaluation
1. Demonstrating focal slowing or conduction block of median nerve fibers across the carpal tunnel
2. Excluding median neuropathy in the region of the elbow
3. Excluding brachial plexopathy predominantly affecting the median nerve fibers
4. Excluding cervical radiculopathy, especially C6 and C7
5. If a coexistent polyneuropathy is present, ensuring that any median slowing at the wrist is out of proportion to slowing expected from the polyneuropathy alone
Nerve Conduction Studies
The nerve conduction strategy for evaluating possible CTS is outlined in Box 17–2. The pathophysiology of CTS typically is demyelination, which, depending on the severity, may be associated with secondary axonal loss. In moderate to advanced cases, the electrodiagnosis usually is straightforward. On routine median studies, a demyelinating lesion at the carpal tunnel results in slowing of the distal motor and sensory latencies. If there is either demyelination with conduction block or axonal loss, the distal compound muscle action potential (CMAP) and sensory nerve action potential (SNAP) amplitudes, stimulating the median nerve at the wrist, will be decreased as well.
Box 17–2
Recommended Nerve Conduction Study Protocol for Carpal Tunnel Syndrome
1. Median motor study recording abductor pollicis brevis, stimulating wrist and antecubital fossa
2. Ulnar motor study recording abductor digiti minimi, stimulating wrist, below groove, and above groove
3. Median and ulnar F responses
4. Median sensory response, recording digit 2 or 3, stimulating wrist
5. Ulnar sensory response, recording digit 5, stimulating wrist
6. Radial sensory response, recording snuffbox, stimulating over the lateral radius
The study is highly suggestive of isolated carpal tunnel syndrome if
No further nerve conductions are necessary, proceed to electromyography (EMG).
Median-versus-ulnar comparison studies
1. Comparison of the median and ulnar mixed palm-to-wrist peak latencies, stimulating the median and ulnar palm one at a time 8 cm from the recording electrodes over the median and ulnar wrist, respectively
2. Comparison of the median lumbrical and ulnar interossei distal motor latencies, stimulating the median and ulnar wrist one at a time at identical distances (8–10 cm), recording with the same electrode over the 2L/interossei
3. Comparison of the median and ulnar digit 4 sensory latencies, stimulating the median and ulnar wrist one at a time at identical distances (11–13 cm) and recording digit 4
Median-versus-radial comparison study
1. Comparison of the median and radial digit 1 sensory latencies, stimulating the median nerve at the wrist and the superficial radial sensory nerve at the forearm one at a time at identical distances (10–12 cm) and recording digit 1
Median segmental sensory study
1. While recording digit 3, stimulate the median nerve at the wrist and in the palm (with the palm-to-digit distance being one-half of the wrist-to-digit distance). Then calculate the wrist-to-palm conduction velocity and compare it to the palm-to-digit conduction velocity
Other important considerations:
1. If there is a co-existent polyneuropathy, the case will be more challenging. The question will be: is the median nerve slowing out of proportion to the slowing associated with the polyneuropathy. It is possible that all the motor and sensory latencies may be prolonged from the polyneuropathy itself. In addition, it would not be uncommon that the sensory and mixed studies may be absent, in which case the palmar mixed, digit 4, and digit 1 comparison studies cannot be used. In this situation, the lumbrical – interosseous comparison is often the most useful internal comparison study, as these motor responses usually remain present in a polyneuropathy.
2. In the unusual situation wherein there is a co-existent ulnar neuropathy at the wrist, all of the median versus ulnar internal comparison studies may be unhelpful, as both the median and ulnar latencies may be prolonged. In this situation, the median versus radial internal comparison study or the median segmental sensory study would be most useful.
3. If there is a co-existent ulnar neuropathy at the elbow (which would not be uncommon), the ulnar mixed and sensory responses may be absent, in which case the palmar mixed and digit 4 studies cannot be used. In this situation, the median versus radial internal comparison study, the median segmental sensory study, or the lumbrical – interosseous comparison would be most useful.
4. If the distal median motor or median sensory amplitudes are low, this may denote either axonal loss or distal conduction block. The only way to differentiate between these two is to stimulate the median nerve in the palm and compare the amplitudes with wrist stimulation. Any palm/wrist ratio >1.6 for sensory and >1.2 for motor amplitudes denotes some conduction block.
Median-versus-Ulnar Comparison Studies
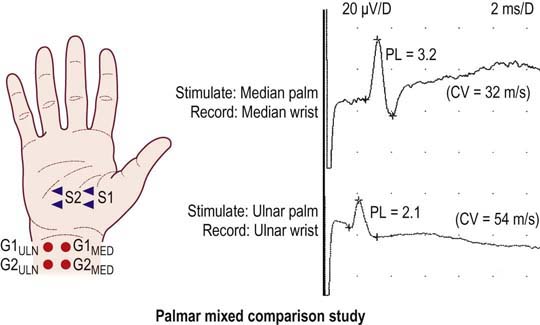
Median-versus-Ulnar Palm-to-Wrist Mixed Nerve Studies
The technique is performed by stimulating the median nerve in the palm, recording the median nerve at the wrist, and comparing it with the ulnar nerve stimulated in the palm and recorded over the ulnar nerve at the wrist (Figure 17–8). Each nerve is stimulated supramaximally in the palm at a distance of 8 cm from its respective recording electrodes. The median nerve is stimulated in the palm on a line connecting the median nerve in the middle of the wrist to the web space between the index and middle fingers. The ulnar nerve is stimulated in the palm on a line connecting the ulnar nerve at the medial wrist (lateral to the flexor carpi ulnaris tendon) to the web space between the ring and little fingers. Supramaximal responses are obtained for each nerve, and the difference between the onset or peak latencies is calculated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree