Figure 34.1. Algorithm for the treatment of intracranial hypertension.
HDB = Barbiturates in high doses; DC = Decompressive craniectomy; HV = Hyperventilation.
Although we argue that the best approach would be personalized treatment of a patient’s cerebral hemodynamics, we refer to the Brain Trauma Foundation’s clinical guidelines. It should be noted that each successive therapeutic step is taken only if a previous step has failed (no control of ICP within 30 minutes of onset), without suspending the former agent but adding a new measure.
Accepted, although not unanimously, is the use of muscle relaxants as a first step to control ICP. The advantages to the use of muscle relaxants in continuous infusion are the control of ICP during tracheal suction manoeuvres, hygiene and patient repositioning, and easier adaptation to mechanical ventilation. The disadvantages are increased length of ICU stay, increased risk of ventilator-associated pneumonia, failure to maintain contact with the patient’s neurological development and, sometimes, neuropathy and myopathy. Juul et al. in a study involving 326 patients failed to demonstrate longer time without ICH in patients who had received muscle relaxants. Therefore, muscle relaxants (e.g., as pancuronium, vecuronium, rocuronium, cisatracurium, etc.) should be reserved for very specific occasions, advising the use of vecuronium by increased hemodynamic stability that its use entails.
First Tier Measures
When ICP is monitored with an intraventricular catheter, drainage of 2-3 ml of CSF through the ventriculostomy catheter reduces ICP, which on many occasions permits the control of ICP without the need to move on to other therapeutic steps. For this purpose, the drain should not be kept open continuously but for as long as required to normalise ICP. In other cases, especially in diffuse lesions with absent cisterns and/or compressed ventricles, this manoeuvre can be useless to normalise ICP. However, its major drawbacks are technical (i.e., inability to insert the catheter into the ventricle if there is displacement of the medial axis) and possible complications: infectious, since the operation and maintenance of the catheter over 4-5 days is associated with a high rate of infection and to a lesser extent (<1.5%) bleeding in the catheter line.
Osmotherapy represents the next therapeutic step. Mannitol is the most widely used agent, followed by hypertonic saline 3%, 7.5% and 24%. Few studies have compared these agents; however, according to some, hypertonic saline can provoke a more intense fall and lasting resolution of ICP, and would therefore be effective when ICH is refractory to other agents, while mannitol would more sharply increase CBF. However, the available evidence is still insufficient to recommend hypertonic saline over mannitol. The agents act through two different and complementary mechanisms. The first and immediate one is hemodynamic, which results in an increase in MAP, CPP and CBF. According to Rosner, it reduces ICP by activating the vasoconstrictor cascade. Another beneficial outcome in relation to its hemodynamic action is the improvement of cerebral microcirculation by reducing blood viscosity.
The second and most studied effect is the creation of an osmotic gradient that causes water to flow across the intact blood-brain barrier. Dehydration of the brain parenchyma facilitates the maximum reduction of ICP observed around 20 minutes post-infusion. When the blood-brain barrier is altered, worsening of edema has been reported with the use of mannitol, as well as an increase in bruises with the use of hypertonic saline. Favourable possible effects of mannitol are the decrease in CSF production and oxygen uptake of free radicals, while hypertonic saline has been reported to decrease endothelial edema and increase cerebral oxygen transport.
Mannitol 20% should be administered in bolus doses from 0.25 to 1 mg/kg as often as needed to be effective, but at least every 4-6 hours to maintain the osmolar gradient and avoid the rebound effect, not to mention that mannitol doses are higher (1 g/kg) when elevated ICP causes brain herniation. The dose of hypertonic saline is not well established, but it is recommended to be sufficient to maintain serum sodium >145 mEq/l. Our group employs bolus 4 ml/kg of 3% sodium, which is repeated as needed and osmolarity does not exceed 320 mOsm/l.
Osmotherapy, particularly when used for long periods, is associated with complications such as dehydration with subsequent hypovolemia, a complication that would buffer the hemodynamic and rheological effects and electrolyte abnormalities, especially hypernatremia, with the use of hypertonic saline, heart failure, bleeding diathesis, and phlebitis. Mannitol also has a toxic effect on the renal tubules, an effect that is greater in hypovolemia, use of nephrotoxic drugs, sepsis or previous kidney disease. Although the toxic action of mannitol is reversible, it is sometimes difficult to manage the patient.
When previous measures have failed, hyperventilation will be initiated, maintaining PCO2 at 30-35 mmHg. Hypocapnia induces a decrease in CBF and thus cerebral blood volume responsible for the decrease in ICP. PCO2 levels below those recommended are dangerous because of the risk of producing ischemia due to both the vasoconstrictor effect of hypocapnia and the leftward shift that tissue alkalosis determines on the hemoglobin dissociation curve (Bohr effect). In our experience, one of the leading causes of cerebral hypoxia, PtiO2 is evidenced by the decrease in PaCO2, which currently prevents the routine use of hyperventilation. Moreover, a study on the effectiveness of prophylactic hyperventilation in TBI showed poorer outcomes in patients at 3 and 6 months after head injury. We must insist on the need to monitor SjO2, or better PtiO2, when hyperventilation is used for sustained treatment of ICH.
Second Tier Measures
The best overall management of TBI has led to a decrease in the number of patients with ICH refractory to conventional medical and surgical measures used to lower ICP. However, in approximately 12% of patients it´s necessary to proceed to second level measures, as refractory ICH is associated with a mortality of 84-100%. Since there is no scientifically established hierarchy in the use of these measures, we will describe them according to our own experience. Although its usefulness was questioned in a systematic review, high-dose barbiturates (HDB) are continuing to be administered now that numerous case studies have shown that these drugs can reduce mortality and decrease ICP in refractory ICH.
The main effects of HBD seem to lie in their property to couple CBF to metabolic demands, so that the drop in metabolic requirements in TBI would be accompanied by a parallel decrease in CBF and cerebral blood volume, thereby inducing a reduction in ICP and an improvement in CPP. Besides their effect on ICP, HDB have been attributed with neuroprotective properties which cause cerebral vasoconstriction which could decrease cerebral metabolism and inhibit lipid peroxidation mediated by oxygen-free radicals.
The most widely used HBD are pentobarbital and thiopental, which must be administered with a loading bolus and an hourly maintenance dosage. The usual loading dose for thiopental is 1.5 mg/kg over 30 minutes and 10 mg/kg over 30 minutes for pentobarbital. The maintenance dose is 1-6 mg/kg for thiopental and 5 mg/kg for pentobarbital. It was once advised to monitor plasma HDB levels to adjust the maintenance dose. However, monitoring HDB levels has not been helpful to avoid side effects nor to optimise their therapeutic effects. Currently, EEG monitoring is the preferred way to adjust the dose, mainly by bispectral index (BIS) monitoring; as they appear in bursts of EEG suppression, the greatest effect on metabolism and CBF is achieved.
Among the adverse effects of HDB the most pernicious is arterial hypotension, which occurs almost normally and should be prevented and treated vigorously, vasoconstriction (or cerebral vasoplegia), and decreased T lymphocyte activity. Arterial hypotension is more frequent in elderly patients with a history of cardiovascular diseases and in patients with hypovolemia, acidosis, hypoalbuminemia or infection. In patients treated with HDB it´s therefore necessary to carry out periodic analysis of plasma albumin, pH, blood gases and continuous MAP monitoring.
A further problem posed by HDB is the difficulty in the early diagnosis of infection because the patient may be hypothermic due to the action of HDB and peripheral leukocytosis may not be detectable. As a general guide, HDB can be more useful in patients with higher ICH when the CT scan shows signs of diffuse brain swelling, moderate hyperventilation reduces ICP, SjO2 >70 mmHg or PtiO2 >20 mmHg, and before a high-velocity TCD exam.
As second-tier therapy in refractory ICH, after the first 24 hours of TBI, and an absence of global ischemia or regional cerebral hypoxia as measured by oximetry (PtiO2 or SjO2), profound hyperventilation can be used (PaCO2 27±2 mmHg). As with HDB, hyperventilation is most useful when the CT shows signs of diffuse brain swelling, CPP >60 mmHg, SjO2 >70 mmHg or PtiO2 >20 mmHg, and TCD ultrasound shows high average velocity flow.
Although performed for over a century for treating ICP, decompressive craniectomy (DC) has attracted renewed and growing interest from groups that advocate it as a first- level procedure. However, controversy persists about its appropriateness. First, not all published series of adult patients have shown improved the results. The only prospective study conducted to date was positive but not statistically significant and involved in 13 pediatric TBI patients and 14 controls: It showed that ICH was reduced and that outcome was better in the patients who had received DC. The prospective, controlled Australian DECRAN study, which has recently been completed, has failed to demonstrate better outcome in the DC-treated group. We are awaiting the results of the European ICP Rescue study to know whether they contradict or otherwise confirm the DECRAN results.
Adding to the controversy over DC is the high rate of substantial complications: hematoma, intracranial cerebral hyperemia with or without associated edema, hydrocephalus, subdural hygroma, secondary heart attack, fungus cerebri and neurological deficits related to bone removal. Furthermore, mixed series can create confusion, since primary DC is not identical or prophylactic after surgery performed on an injury mass and cannot be compared with a secondary DC performed when other measures fail to control ICP.
Nonetheless, there is consensus that craniectomy in the treatment of refractory ICH should take place within the first 48 hours, with wide resection (15 cm x 15 cm), followed by duroplasty, bilateral in diffuse lesions and unilateral in mass lesions, and that the beneficial effect will depend on the amount of bone removed from the temporal base when the total size equals that of the mass lesion. Young patients without extremely elevated ICP, not at risk of dying or poor outcome, could benefit more from DC.
Increasing MAP, or the CPP-oriented strategy, is another second level option. It is based on the theory advanced by Rosner (Figure 34.2) that in TBI self-regulation is never lost but rather is shifted to the right, the critical point on the autoregulation curve, requiring, therefore, greater MAP to induce vessel narrowing. According to the author, the decrease in CPP initiates a cerebral vasodilator cascade that increases the cerebral blood volume, which, in turn, leads to an increase in ICP, thus closing a vicious circle.
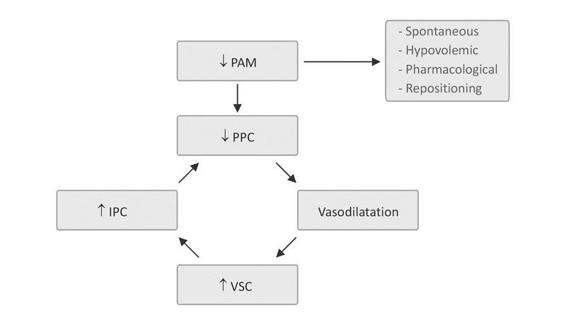
Figure 34.2. Rosner vasodilator cascade.
In contrast, an increase in CPP through a decrease in ICP or an increase in MAP would trigger a beneficial vasoconstrictor cascade that reduces cerebral blood volume and controls ICP. To lower ICP, MAP should be gradually increased to a value that occurs in the fall of ICP. MAP values may sometimes be as high as 150-160 mmHg and sometimes do not lead to such a reduction in ICP, despite having exceeded these values. This expands the intravascular volume with saline. If MAP does not increase despite good vascular filling with fluids or its value is insufficient to trigger the vasoconstriction cascade, the infusion of vasoactive agents such as norepinephrine or phenylephrine may be an option for the stated objectives.
Renal failure is the most common complication when high-dose vasoactive drugs are administered to increase MAP. At this point, increasing MAP is usually reserved for patients with space-occupying lesions in which previous options have failed and there are no systemic contraindications to increasing MAP.
Another measure that appears and reappears as an option in second level treatment is controlled hypothermia. Its interest resides in the neuroprotective effect of controlled hypothermia. At the most studied temperatures (32-34°C) a reduction in systemic and cerebral demands of oxygen is reportedly coupled with a decrease in CBF and ICP, decreasing the release of excitatory amino acids and protecting against free radicals. At lower temperatures (<32°C), however, hypothermia has been associated with immunosuppression, hypokalemia (with severe hyperkalemia during overheating that can result in cardiac arrest) and severe coagulation disorders. Studies involving small TBI populations, especially those by Marion in 1999, reported beneficial effects of hypothermia on outcome; however, a well-designed prospective and controlled study by Clifton in 2001 failed to demonstrate efficacy. Recently, the good results in neurological recovery obtained in patients resuscitated after sudden death from ventricular fibrillation has rekindled interest in hypothermia.
A recent meta-analysis of 13 studies involving more than 1300 patients with TBI, although mixing dishomogeneous data sets and procedures, reported a modest reduction in mortality and a slightly higher percentage of favourable outcomes. However, complications, especially pneumonia, may outweigh the neurological benefit. Therefore, pending the availability of new controlled studies, cautious use is recommended in centres with expertise in selected cases, with temperatures no lower than 33°C, with slow heating (>48 hours).
A different approach to the clinical guidelines issued by the Brain Trauma Foundation and the European Brain Injury Consortium is advocated by the school in Lund (Sweden). Its main objective is to control ICP, assuming that any increase results from vasogenic edema caused by an impaired blood-brain barrier and loss of cerebral autoregulation. The increase in MAP would be the force that in these pathophysiological conditions produces cerebral edema. To abort this cascade, MAP must be reduced, oncotic plasma increased and the venous vascular space collapsed with dihydroergotamine. Despite the good results reported by the Swedish authors, the shortness of the series and the lack of a control group currently make this therapeutic strategy unacceptable to the scientific community. Table 34.12 illustrates the Lund therapy protocol.
General measures | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




