Chapter 127 Mesencephalic Tractotomy and Anterolateral Cordotomy for Intractable Pain
Mesencephalic Tractotomy
Anatomic Background
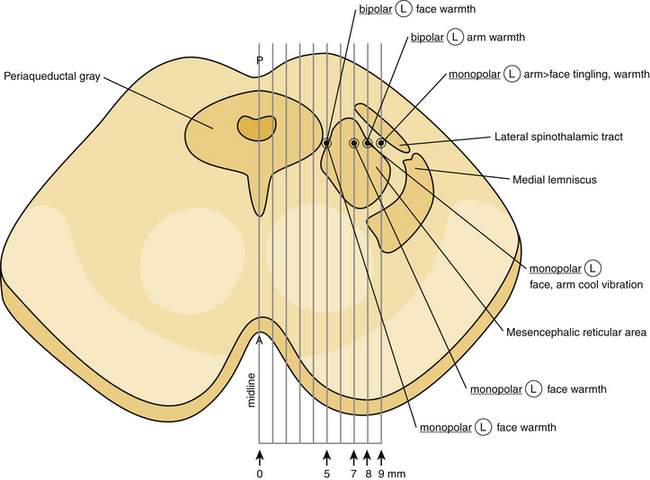
It is well known that the mesencephalon is topographically divided into three distinct regions: tectum, tegmentum, and basal portion.1 The tectum, which is a mixture of gray and white matter, is located dorsally to the central gray matter in cross-section, occupying the dorsal surface of the midbrain. It includes the superior and the inferior colliculi. The tegmentum constitutes the main, central part of the mesencephalon and lies anterior to the central gray. It contains all the ascending and descending neuronal tracts, including the brachium conjuctivum (superior cerebellar peduncle), the rubrospinal tract, the medial lemniscus, the trigeminal lemniscus, the spinothalamic and spinotectal tracts, the medial longitudinal fasciculus, the central tegmental tract, the lateral lemniscus, and the dorsal longitudinal fasciculus. The brachium conjuctivum represents a massive bundle of fibers arising from the deep cerebellar nuclei, decussating at the level of the inferior colliculus in the tegmentum and terminating on the red nucleus and the ventrolateral thalamic nucleus. The rubrospinal tract lies anteriorly to the brachium conjuctivum and conveys fibers from the red nucleus to the spinal cord and the inferior olive. The medial lemniscus lies lateral to the brachium conjuctivum, and conveys kinesthesia and discriminative touch from the spinal cord to the thalamus. The fibers of the medial lemniscus are somatotopically organized with the cervical fibers being more medial, while sacral fibers are more lateral.1 The trigeminal lemniscus lies medially to the medial lemniscus. The spinothalamic and spinotectal tracts lie laterally to the medial lemniscus and convey pain and temperature sensation from the contralateral side of the body. The fibers of the spinothalamic and spinotectal tracts are somatotopically organized with cervical fibers being more medial, while sacral most lateral (Fig. 127-1). The medial longitudinal fasciculus lies dorsally to the brachium conjuctivum. The central tegmental tract lies dorsally to the brachium conjuctivum and laterally to the medial longitudinal fasciculus, conveying fibers from the basal ganglia and the mesencephalic nuclei to the inferior olive. The lateral lemniscus lies dorsally and laterally to the spinothalamic tract, conveying auditory fibers. The dorsal longitudinal fasciculus (fasciculus of Schutz) is a periaqueductal ascending and descending fiber system arising from the hypothalamus and terminating to the autonomic nuclei of the pons and the medulla, conveying autonomic fibers.
The basal portion of the mesencephalon includes the cerebral peduncles and the substantia nigra, a pigmented nuclear mass, which lies between the dorsal surface of the cerebral peduncle and the tegmentum.1 In addition, several nuclear groups can be found in the mesencephalon at the level of the inferior colliculi. These include the mesencephalic nucleus of the trigeminal nerve, the nucleus of the trochlear nerve, the interpeduncular nucleus, the nucleus parabrachialis pigmentosus, the dorsal tegmental nucleus, the ventral tegmental nucleus, the pedunculopontine and the lateral dorsal tegmental nuclei, the dorsal raphe nucleus, the parabigeminal nucleus, the nucleus pigmentosus (locus ceruleus), and the substantia nigra. The red nucleus, the oculomotor nucleus including the Edinger-Westphal and the Perlia’s nuclei, the interstitial nucleus of Cajal, the rostral interstitial nucleus of the medial longitudinal fasciculus, the Darkschewitsch’s nucleus, and the nucleus of the posterior commissure can be found at the level of the superior colliculi (Fig. 127-2).
The central gray of the mesencephalon represents a complex anatomical structure. It surrounds the Sylvian aqueduct and contains several nuclei and a mixture of myelinated and unmyelinated fibers.1 It is well known that the central gray is implicated in pain conduction. Enkephalin, a neuropeptide associated with pain alleviation, has been identified in large quantities in the central gray. It has been postulated that stimulation of ventrolateral regions of the central gray produces enkephalins, which subsequently act on serotonergic neurons in the medulla oblongata, which in turn project on afferent axons (concerned with pain) in the dorsal horn of the spinal cord to produce analgesia. Contrariwise, stimulation of the lateral and rostral areas of the central gray facilitates pain conduction. Furthermore, the production of several other neuropeptides by different areas of the central gray, such as neurotensin, substance P, cholecystokinin, serotonin, somatostatin, and dynorphin has been demonstrated in numerous studies.1 The central gray has been also involved in vocalization process, modulation of medullary respiratory centers, vertical gaze, aggressive behavior, and control of reproductive behavior.
Historical Evolution
The first open mesencephalic tractotomy for managing medically intractable pain was performed by Dogliotti in 1938.2 Although he described his surgical procedure as a surgical section of the “lemniscus lateralis” at the level of the rostral pons, it should be considered certain that the level of the incision was at the lower mecencephalon (inferior colliculi).2,3 His surgical methodology consisted of surgical exposure of the lower part of the midbrain and introduction of an electric coagulator into the lateral sulcus.2 His initial series included four patients with medically refractory pain.2 He reported pain abolishment postoperatively in two of them, while there was significant pain reduction in one patient, and the other patient died immediately after surgery.2 In all his reported cases, there was some degree of hemihypoalgesia associated with paresthesia postoperatively.2
Bailey and his coworkers described their experience from performing an open mesencephalic tractotomy for managing medically intractable pain.3 They performed their tractotomy by making an incision, approximately 8 mm in length and 4 mm deep, extending from the lateral sulcus of the midbrain to the posterior edge of the inferior colliculus, on a plane between the superior cerebellar artery and the trochlear nerve.3 They reported excellent pain control postoperatively, while they observed some transient postoperative drowsiness and aphasia in one of their cases, most probably due to surgical manipulation of the dominant hemisphere of their patient.3
Similarly, Drake and McKenzie reported their experience from a series of six patients, undergoing mesencephalic tractotomy due to drug-resistant upper extremity or facial pain.4 In all their cases, the patient was in a sitting position and the incision was made through a small occipital bone flap, placed between the sagittal sinus medially and the transverse sinus inferiorly. The overlying occipital lobe was gently retracted superiorly and laterally, and the tentorium was incised along the straight sinus. The posterolateral aspect of the midbrain was exposed after opening the arachnoid overlying the cisterna ambiens. A tractotomy incision was placed by using a cordotomy knife. The incision usually extended from the lateral sulcus to the lower pole of the superior colliculus, and it was 5 mm deep. They reported that all their patients had no pain and temperature sensation in the contralateral half of the body, postoperatively. However, all their patients developed progressively quite bothersome dysesthesias after surgery, while one patient developed postoperatively severe burning, dysesthetic pain. Interestingly, touch, vibration, and position sensations remained unaffected after surgery in all their cases. They observed in five sixths6 of their cases temporary complete homonymous hemianopsia postoperatively, apparently due to the retraction of the occipital lobe and/or the sacrifice of the cortical occipital veins. The observed hemianopsia was spontaneously resolved with no further sequelae.4
Stereotactic mesencephalotomy for pain management was introduced by Spiegel and Wycis in 1947.5,6 In their first attempts, they combined this procedure with thalamotomy for treating patients with chronic, medically refractory pain of noncancerous origin, which showed no response to previous surgical procedures, such as retrogasserian rhizotomy, sympathectomy, and cordotomy.5,6 In their initial cases, they aimed not only at the spinothalamic and the quintothalamic tracts, lateral to the Sylvian aqueduct, but also the tegmentum adjacent areas, dorsally to the red nucleus.6 Pneumoencephalography was routinely employed for the surgical planning. Both patients in their initial report demonstrated satisfactory long-term pain relief.5,6
In a later study, they described their modified technique, in which the entry point was through a burr hole, placed on a paramedian plane (7.5 mm lateral to the midline) at the interaural level, and the trajectory was inclining 34 degrees posteriorly, passing through the posterior commissure, which was serving as a reference point.7 A lesion, approximately 5.5 to 9.5 mm in dorsoventral diameter, was placed 2 to 3 mm posterior and 0 to 3.5 mm inferior to the posterior commissure, destroying the spinothalamic and the reticulothalamic tracts at this level, after applying anodal direct electrical current for 60 seconds. Intraoperative electrical stimulation was usually employed in order to physiologically verify the anatomical target and thus minimize the possibility of any procedure-related complications. They reported immediate complete pain relief in 72.2% (39/54 patients) and partial relief in 31%, while 20.3% demonstrated no response.7 In the case of proximity of the inserted electrode to the deeply sited oculomotor fibers, ipsilateral ocular movements were observed, while tinnitus could occur due to stimulation of the adjacent lateral geniculate body in cases of laterally placed electrode. Their indications included atypical or postherpetic facial pain, pain secondary to tabes dorsalis, chronic pain secondary to spinal cord and spinal roots trauma, cancer-related pain, and thalamic and phantom-limb pain.7 They reported a 7.4% (4/54 patients) mortality rate, limited to patients with thalamic or cancerous pain. They also reported 14.8% permanent postoperative dysesthesia, while ocular disturbances occurred in 3.7%, and permanent motor deficits in 1.8%.7
Torvik reported Leksell’s experience with stereotactic mesencephalic tractotomy (STM).8 They performed their procedures under local anesthesia, while the Leksell stereotactic apparatus was used.8 A small burr hole was placed in the parieto-occipital region and an electrode was stereotactically inserted utilizing the Sylvain aqueduct and the posterior commissure as reference points.8 An electrical stimulation study was routinely performed for verifying the anatomic target, and subsequently a spherical lesion was placed via a bipolar coagulation (lesioning temperature 52 to 60 °C), extending from the level of posterior commissure to the rostral level of the trochlear nucleus in the rostro-caudal direction. A microscopic examination of the lesioned midbrain area in a postmortem autopsy study in their patients, showed a sharply circumscribed cavity filled with debris and macrophages surrounded by a thin and sparse gliotic rim, with no evidence of retrograde or anterograde corticospinal fiber degeneration. Interestingly, the ipsilateral medial lemniscus and the spinothalamic tract were completely destroyed; the adjacent reticulothalamic tract was massively damaged, while the superior colliculus and the red nucleus were partially destroyed. Torvic reported in his article that despite the extensive lesioning of the reticulothalamic and spinothalamic tracts at the midbrain, there was still postoperatively some residual pain sensation. He postulated that tracts other than the reticulothalamic and spinothalamic may be involved in pain propagation.8
Orthner and Roeder9 reported their surgical technique in stereotactic mesencephalic tractotomy in managing patients with trigeminal, postherpetic, phantom-limb, and Dejerine-Roussy syndrome pain. Their anatomic target was at the level of the posterior commissure, 7.5 mm lateral to the midsagittal plane.9 They routinely employed an electrode and a radiofrequency generator at 30 mA for 30 seconds for making a lesion. They emphasized the importance of partially destroying the spinoreticular along with the spinothalamic tract, in order to maximize the pain relief effect.9
Mazars et al.10 reported in 1976 their experience with a different and more accurate approach (through a transcortical posterior parietal lobe trajectory) for mesencephalic stereotactic tractotomy (MST). The proposal of a more precise anatomic target in their clinical series had as a consequence equally good pain relief rates with significantly reduced morbidity.10 The occurrence of postoperative dysesthesia and anesthesia dolorosa was significantly diminished; on the other hand, the occurrence of oculomotor palsies was increased due to the passage of the lesioning electrode through the quadrigeminal plate.10
At approximately the same time Amano et al.11 reported their technique and their results from treating medically intractable pain of cancerous origin with MST. They performed their procedures under local anesthesia by utilizing either Sano’s or Todd-Wells’ stereotactic head frame.11 The surgical planning was based on pneumoencephalography and the Schaltenbrand-Bailey stereotactic atlas.11 The dorsal portion of mesencephalic tegmentum near the central gray at the level of the rostral end of the superior colliculus was used as anatomic target. The stereotactic coordinates for their selected target was P 14, H –5 to –8, and L 5 to 8, while the laterality of the target was measured from the midline of the Sylvain aqueduct.11 They emphasized that the usage of frontal entry point could minimize the incidence of Parinaud’s syndrome postoperatively. A radiofrequency lesion was made after electrophysiologic confirmation of the target. They reported significant information regarding the electrophysiologic profile of the mesencephalic reticular formation by employing detailed microelectrode recordings.11
Nashold and his coworkers employed a slightly different surgical technique for performing stereotactic mesencephalic tractotomy.12–15 The surgical planning was based on a contrast ventriculography for visualizing the Sylvian aqueduct and the anterior (AC) and posterior commissures (PC). The procedure was routinely performed under local anesthesia. A burr hole was placed 1 cm anterior to the coronal suture and 1.5 cm from the midsagittal plane. Nashold et al. had developed a mesencephalon stereotactic atlas that could be used for this anterior approach.12–15 After accurate recognition of the rostral end of the Sylvian aqueduct and the AC-PC line, an electrode was inserted in a trajectory crossing the AC-PC line at a 65- to 70-degree angle. The lateromedial angulation of the electrode was 2 to 4 degrees off the midsagittal plane. Electrical stimulation could be performed for verifying the anatomic target and identify the fine somatotopic organization of the spinothalamic and reticulothalamic tracts. A radiofrequency generator was used for 30 seconds for placing a spherical lesion. Nashold initially in his series utilized a target located at the level of the PC, 3 mm posterior to that (“superior colliculus target”), while later he utilized a target located 5 mm posterior, inferior, and lateral to the PC (“inferior colliculus target”).12–15 They greatly emphasized in their reports the importance of intraoperative stimulation for verifying the anatomic target and thus maximizing the physiologic effect.12–15
Indications for Stereotactic Mesencephalic Tractotomy
The recent advances in neuropharmacology, the development of multidisciplinary pain management strategies, and the emerging of novel neuromodulative surgical techniques have limited the role of SMT, making it a valid treatment option only for patients with medically refractory pain due to extensive carcinoma involving the head, neck, and/or arm, with likely survival time on the order of 6 (±3) months. Several previous reports confirm pain relief and also pain-associated anxiety after performing SMT and lesioning the spinoreticular along with the spinothalamic tracts in cases of extensive head and neck cancer.9,12 The performance of SMT carries the advantage of reducing the patient’s emotional reaction to pain. It has been reported that the postoperative calming effect of the patient’s fear and anxiety after a SMT closely resembles that of bilateral cingulotomy.12
In cases of unilateral facial, neck, and/or arm medically intractable pain of cancerous origin, a SMT on the opposite side should be performed. In cases of bilateral pain, the side of the greatest involvement should be managed. Whisler and Voris reported no serious postoperative side effects from placing bilateral SMT lesions in patients with bilateral pain.12,13
Patients suffering from phantom-limb pain following avulsion of the brachial plexus, postcordotomy dysesthesia, or central pain secondary to thalamic syndrome, who failed medical or other surgical treatment, may be candidates for SMT. Although the analgesic effect of SMT wears off in the vast majority of the reported cases there are reports of long-lasting pain relief.9
Surgical Technique
Preoperative Preparation
The surgical procedure is carefully explained to the patient. The importance of the patient’s cooperation cannot be overemphasized. The patient remains fasted for a minimum of 8 hours before the procedure. The required dose of analgesics is administered parenterally. Antibiotics are begun intravenously on the morning of surgery and are continuing for 24 hours after surgery. Midazolam is given intravenously for preoperative sedation. The procedure is routinely performed with neuroleptanalgesia using intravenous alfentanil and propofol. The area of the burr hole is clipped and saved. The skin is thoroughly prepped and appropriately draped in a standard sterile fashion. A field block of the scalp is performed, and then a mixture of 20 ml of 0.25% bupivacaine hydrochloride with epinephrine, 20 ml of 0.5% lidocaine with epinephrine, and 4 ml of sodium bicarbonate 8.4% is used.17 The bicarbonate neutralizes acidity and considerably reduces the discomfort of the block.
Operative Procedure
The MRI/CT to compatible Leksell base ring and localizer (Elektra, Stockholm, Sweden) are fixed to the patient’s skull.17 Carbon fiber posts and MRI/CT-compatible pins are used. The MRI scan consists of a contrast enhanced T-1–weighted volume acquisition and MPRAGE pulse sequences, using axial 1.3-mm slices with zero slice gaps. This is followed by a whole head CT scan, using 3-mm slices with zero slice gaps. The two data sets are imported over the local network to the computer workstation. After fusing the MRI data to the CT data, targets, and trajectories are defined. If an automatic fusion software program is not available, a visual anatomic point-to-point matching between CT and MRI studies may be performed for ruling out any magnetic field-generated distortion. A probe-view algorithm is used to maximize the distance between any surface cortical veins and electrode at the cortical entry points.
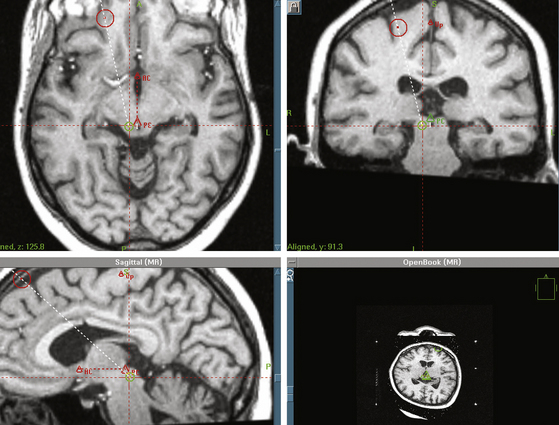
After defining the anterior commissure (AC), the posterior commissure (PC), and the AC-PC line, the mesencephalic target is set 5 mm inferior to the AC-PC line, and 5 to 8 mm lateral to the midsagittal plane, at the level of the superior colliculus17 (Fig. 127-3). The entry point and a transcortical trajectory are selected, and a frontal stab incision is appropriately made. A 7/64-inch twist–drill hole is usually placed, approximately 1 cm anterior to the coronal suture and 1.5 cm lateral to the midsagittal plane. The underlying dura and pia are carefully cauterized with monopolar cautery. A 1.1-mm diameter bipolar straight stimulation-lesioning thermo-coupled electrode (F.L. Fischer, Freiburg, Germany) (Fig. 127-4) is inserted and proper positioning is confirmed with intraoperative fluoroscopy. Neuroleptanalgesia must be reversed as much as possible, at this point, in order to maximize patient cooperation. Stimulation studies may be performed at this point for physiologic verification of the anatomic target (Fig. 127-1).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree