19 According to statistics from the American Cancer Society more than 1.2 million people are diagnosed with cancer each year. Some time after their diagnosis 20 to 30% of these patients will develop symptoms related to bone metastasis, and more than 70% will have evidence of bone metastasis at autopsy. Breast, prostate, and lung cancers are the most common primary tumors that spread to bone; however, hematologic malignancies such as myeloma and Hodgkin’s disease also frequently spread to these structures (Table 19-1). As advances in the treatment of these primary tumors evolve and increase the length of survival, the incidence of bone metastasis will also rise. Improvements in neoadjuvant treatment strategies have improved local-regional control; therefore, patients with distant metastatic disease are surviving longer, and control of the metastatic disease plays a significant role in the their overall prognosis. Early diagnosis and management of these lesions become more important not only in improving survival but also in controlling pain and preserving quality of life.
Metastatic Tumors of the Spine and Spinal Cord
| Primary site | % of all spine metastases (n =11,884) |
| Breast | 30.2 |
| Lung | 20.3 |
| Blood | 10.2 |
| Prostate | 9.6 |
| Urinary tract | 4.0 |
| Skin | 3.1 |
| Unknown primary | 2.9 |
| Colon | 1.6 |
| Other | 18.1 |
The most common sites for bone metastasis are the spine and axial skeleton.1,2 Most of these include disease of the vertebral body with or without epidural compression. Tumor, however, can spread intradurally, resulting in either intramedullary spinal cord metastasis (ISCM) or extramedullary lesions.
 Extradural Metastasis
Extradural Metastasis
Pathophysiology of Bone Metastasis
Tumor cells predominantly metastasize to heavily vascularized skeletal structures (red bone marrow) such as vertebral bodies, ribs, or proximal long bones.1 The predilection to metastasize to a specific vertebral region is based on the volume and number of vertebral bodies; thus, 70% of metastases are found in the thoracic spine, 20% in the lumbar spine, and 10% in the cervical spine.
Blood Supply
The high propensity for metastatic vertebral body involvement is related to physiologic properties of blood flow. Batson’s plexus has been implicated in the wide dissemination of prostate cancer to the spine and accounts for the high percentage of vertebral body disease as opposed to posterior element involvement (Fig. 19-1).1 This high-volume, low-pressure, valveless system of veins communicates with the pelvic plexus and intercostals veins. Arterial spread has also been implicated as a potential cause. In several animal studies the intracardiac injection of tumor cells resulted in patterns of metastatic tumor similar to those seen in clinical scenarios. 3–5 This tumor embolization may explain the predilection for bone metastases to occur at the terminal end of major feeding arteries that supply the bone6,7 and may be the route for dissemination to the posterior spinal elements (i.e., lamina and pedicles, Fig. 19-2).
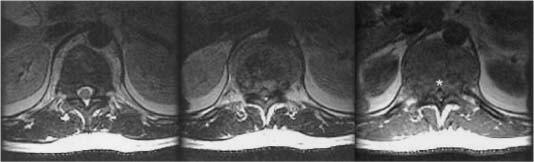
FIGURE 19-1 Axial magnetic resonance images (MRI) of a patient with multiple myeloma with isolated disease in the vertebral body. The posterior longitudinal ligament is intact but displaced by tumor giving rise to the classic V sign (*).
Seed and Soil Theory
Venous drainage patterns with direct dissemination from the primary site explain the distribution of metastases in 30% of cases.8 However, certain tumors such as lung, prostate, and breast have a strong predilection for metastasizing to bone. In 1889 Paget9 proposed the “seed and soil” theory of distant metastases. Considerable research has now been conducted to elucidate the mechanisms of distant spread and the creation of osteolytic and osteoblastic metastases.
Tumor dissemination is a multistep process termed the “metastatic cascade” and involves specific tumor and host-tissue interactions via specific molecular determinants (Fig. 19-3). Angiogenesis enables the growth of primary tumors, tumor cells to access the systemic circulation, and the growth of micrometastatic deposits into large macrocellular lesions. Cell adhesion molecules allow tumor cells to attach to other tumor cells and to extracellular structures such as the basement membranes of blood vessels. Examples of cell adhesion molecules include laminin, integrins, and E-cadherin.4,10–21 This adherence allows the local release of proteolytic enzymes, such as matrix metalloproteinases and type IV collagenase. These enzymes allow tumor cells to cross basement membranes, to gain access to intravascular spaces, and to exit these vessels at distant sites.22–28 Cellular proliferation is mediated by growth factors and uncoupling of regulated mechanisms of cell growth and suppression, angiogenesis, and bone turnover.4,10–26 Only 1% of tumor cells that reach the intravascular space survive; even fewer are able to establish a foothold at a distant site and to grow into a micrometastatic deposit.8
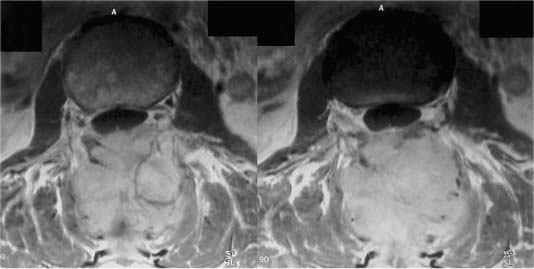
FIGURE 19-2 Axial MRIs of a patient with a neuroendocrine tumor involving only the posterior elements.
FIGURE 19-3 The metastatic cascade.
Osteolysis resulting in bone destruction does not appear to be simply a mechanism of tumor replacing bone but, rather, activation of osteoclasts causing dysregulated remodeling of bone.29 This mechanism is well elucidated in a breast cancer model where parathyroid hormone-related peptide (PTH-rP) released by tumor cells was found to stimulate osteoclastic bone resorption.1 Local release of tumor growth factor B (TGF-B) also may increase the activity of PTH-rP. Other cytokines, notably interleukin 6 (IL-6), also enhance PTH-rP activity by increasing the activity of osteoclast progenitor cells.30–33
Osteoblastic metastasis, notably as a result of prostate cancer, arises through the stimulation of osteoblasts by several putative factors. These factors include TGF-B, fibroblast growth factors, prostate-specific antigen (PSA), and bone morphogenic protein.34–53
Contiguous Spread
Another pattern of spine involvement is contiguous spread from the growth of surrounding soft tissue masses such as paraspinal or chest wall lesions. This pattern is most often seen in neuroblastomas, sarcomas, and lung cancer, particularly in patients with superior sulcus tumors. Tumors can grow through the neural foramen and involve the epidural space. This pattern causes spinal cord compression without necessarily involving surrounding bony structures. These patients often become symptomatic with neurologic deficits without significant complaints of prodromal pain or with pain that is attributed to paraspinal or rib lesions but without significant back pain. Metabolic imaging studies such as positron emission tomography (PET) or radionucleotide bone scans often show no evidence of spinal disease, thus demonstrating the importance of magnetic resonance imaging (MRI) in patients with a history of cancer and unexplained neurologic deficits or significant changes in their pain patterns (Fig. 19-4).
Solitary Bone Lesions
Metastatic dissemination to bone usually occurs with multiple synchronous lesions. Isolated metastases to the vertebral body are rare and should be differentiated from primary bone tumors. The most common tumors to manifest as solitary vertebral body lesions are thyroid, renal, and plasmacytomas. With rare exceptions, patients with solitary metastases eventually develop widespread tumor.54–56
Diagnostic Evaluation
A careful history and physical examination are always paramount. Patients should be questioned about a remote history of cancer. Tumors such as breast and melanoma can have a long dormant period between the appearance of the primary tumor and the development of metastasis. Special attention should be given to the features of the patient’s pain, which plays an important role in the treatment algorithm. There are three categories of pain. First, pain that is worse at night or at rest and typically improves with steroid administration is consistent with biologic pain. Second, classically, radicular pain is described as a band-like area of pain around the chest and thorax, or it assumes a typical radicular pattern when the lesion is in the cervical or lumbar spine. Third, pain that is exacerbated by weight bearing or movement and relieved at rest is pathognomonic for mechanical pain. Biologic pain usually responds well to steroids and radiation. The other two types, however, respond only marginally to nonoperative measures.
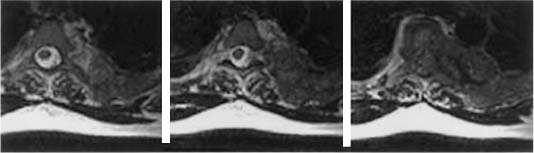
FIGURE 19-4 Axial MRIs of a patient with recurrent breast cancer with a paraspinal mass that enters the spinal canal through the neuralforamen, causing spinal cord compression without significant bony involvement.
Special attention must also be given to a history of recent treatment such as chemo- or radiation therapy. These therapies significantly affect a patient’s ability to undergo and recover from an operation, especially in terms of immunosuppression, coagulation abnormalities, and wound healing.
The physical examination should concentrate on breast, prostate, thyroid, and cutaneous lesions. The laboratory evaluation should include a basic screening panel with a complete blood cell count to assess the presence of myelosuppression and thrombocytopenia. Calcium, phosphorus, and alkaline phosphatase levels assess the degree of bone turnover and identify hypercalcemia associated with malignancy. Liver function tests and erythrocyte sedimentation rates also may be helpful. The level of PTH is used to assess the presence of metabolic bone disease. Urine and serum protein electrophoresis may identify multiple myeloma and β2-microglobulin for lymphoma. Tumor markers, most prominently PSA and CA 125, assess prostate and breast cancer, respectively. N-telopeptide and urine deoxypyridinoline are biochemical markers that reflect bone turnover that may be associated with bone metastases. We, however, do not routinely use these tests. In the future, blood and urine may be screened by polymerase chain reaction to assess for small numbers of tumor cells that could place patients at risk for developing metastatic tumor.
Standard radiographic imaging for patients with a solitary vertebral body metastasis includes computed tomography (CT) scans of the chest, abdomen, and pelvis, and radionucleotide bone scans. These scans help identify a primary site or other sites that may be more accessible for biopsy. This evaluation protocol identifies the primary in ~85% of cases, most commonly renal or lung carcinoma. Increasingly, whole-body 18-fluorodeoxyglucose (FDG)-PET is also being used to assess for other tumor foci and has shown promise with breast, thyroid, and lung tumors.57 Radioiodinated metaiodobenzyl guanidine scans to identify neuroblastomas and octreotide scans to identify neuroendocrine tumors have also been helpful in selected cases.
Needle Biopsy
Solitary lesions involving the vertebral body require a biopsy to establish the diagnosis firmly. Percutaneous CT-guided needle biopsies can be performed through a transpedicle approach (cervical, thoracic, or lumbar) or through the costovertebral articulation (thoracic spine) with minimal complications. In patients with osteolytic lesions, the success rate is ~88%. To obtain core biopsies, we use a 15-gauge osteocut needle (C. R. Bard; Murray Hill, NJ) as an outer sheath through which is passed an 18-gauge percucut needle (C. R. Bard). Osteoblastic lesions, such as prostate and occasionally lymphoma, tend to be more technically challenging. In addition, the rate of diagnostic biopsy tends to be significantly lower in cases of infection.
An inability to establish the diagnosis with a percutaneous biopsy often requires an open biopsy. An open biopsy is best done through transpedicle targeting of the area of the vertebral body exhibiting the greatest change, often with the use of fluoroscopy, image guidance, or both. For patients with paraspinal masses in the chest, thoracoscopic biopsies have been extremely useful. The hypointensity seen on T1-weighted MRIs and thought to be a tumor actually may be bone edema related to a small tumor nodule. Occasionally, 18-FDG-PET has been useful in directing a biopsy to the most metabolically active portion of the vertebral body (Fig. 19-5).
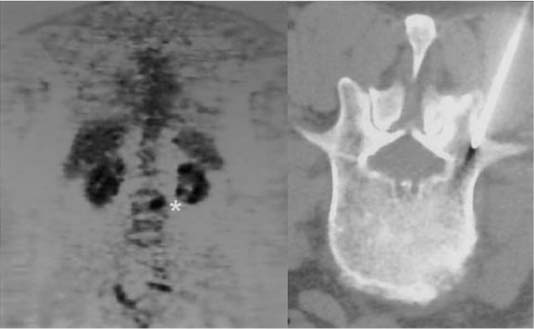
FIGURE 19-5 18-FDG-PET scan showing “hot” area (*) used to target a small lytic lesion seen on CT-guided needle biopsy.
Surgery
The indications for surgical intervention vary and must be individualized based on the patient’s overall medical condition, systemic tumor load, neurologic function, symptoms, and history of prior radiation. The goals for the surgical treatment of these lesions include histologic confirmation, decompression of spinal cord and nerves, spinal column stabilization, and cytoreduction. Further aspects of the surgical management of these lesions are addressed in other chapters in this book.
 Intradural Extramedullary Metastases
Intradural Extramedullary Metastases
The most common type of intradural extramedullary tumor dissemination is the result of leptomeningeal disease, which is discussed in the next section.
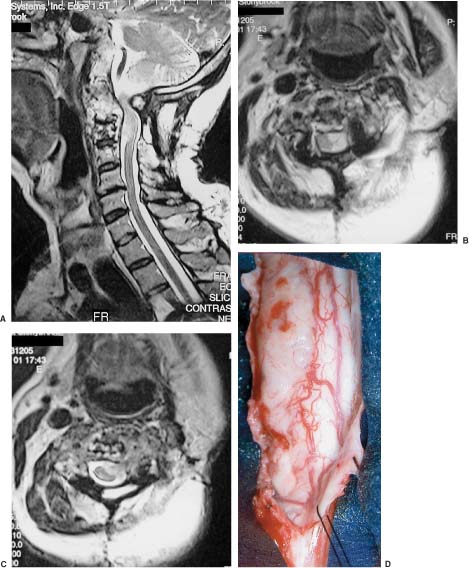
In addition, certain primary central nervous system (CNS) tumors involving the posterior fossa such as medulloblastomas, primitive neuroectodermal tumors, and germinomas, have a high predilection for dissemination through cerebrospinal fluid (CSF) and spinal seeding that results in extramedullary metastasis; however, extramedullary metastasis with non-CNS tumors is exceptionally low. When these lesions do occur, they usually follow a previous surgical intervention in which the dural barrier was violated; however, tumors can also access the intradural compartment by tracking along nerve roots as they exit the thecal sac (Fig. 19-6).
Leptomeningeal Metastases
Leptomeningeal metastases occur in 3 to 8% of all cancer patients.58 The most common solid tumors are breast carcinomas, non-small cell lung cancers, and melanomas. Hematologic malignancies, such as acute lymphocytic leukemia and lymphoma, are also frequently encountered.
Patients with a leptomeningeal tumor often become symptomatic with signs and symptoms reflective of neurologic involvement throughout the neuraxis, such as multilevel radiculopathy.59,60 The most common manifestation is lower motor neuron weakness (more than 80% of patients), followed by reflex loss and dermatomal sensory changes. Symptoms of high intracranial pressure (headache and emesis) and back pain are common. Less frequently, patients manifest with cranial neuropathies or symptoms referable to focal brain involvement (e.g., aphasia, hemiparesis, or hemianopsia). Nuchal rigidity and mechanical signs occur in less than 15% of patients. Although rare, we have seen patients become symptomatic with acute myelopathy from leptomeningeal metastases in the absence of an epidural tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





