and Colin L. W. Driscoll2
(1)
Department of Otolaryngology – Head and Neck Surgery, Stanford University, Stanford, CA, USA
(2)
Department of Otorhinolaryngology, Mayo Medical School, Rochester, MN, USA
Approach to the Internal Auditory Canal
Concept
Retract the temporal lobe in an extradural fashion, and drill through the petrous apex to reach the posterior cranial fossa and internal auditory canal
Conditions treated
Small vestibular schwannoma (intracanalicular lesions and those with a diameter in the cerebellopontine angle <1.0 cm)
Facial nerve decompression or repair
Risks
Retraction injuries to the temporal lobe can cause contusion, edema, or stroke.
Temporal lobe seizure.
Anomic aphasia.
Hearing loss if the labyrinth is violated or auditory nerve involved with the tumor.
Facial nerve injury, particularly if the geniculate ganglion is dehiscent and not recognized.
Carotid artery injury along the horizontal segment.
Dry eye if the GSPN is transected.
CSF leak.
Benefits
Best approach for hearing preservation when removing a vestibular schwannoma that extends to the fundus
Only way to access the labyrinthine course of the facial nerve while preserving hearing
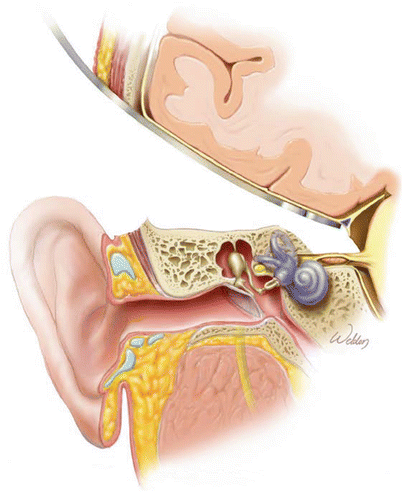
1.
Coronal view of the temporal bone. An intracanalicular vestibular schwannoma is shown. The facial nerve lies superior to the tumor.
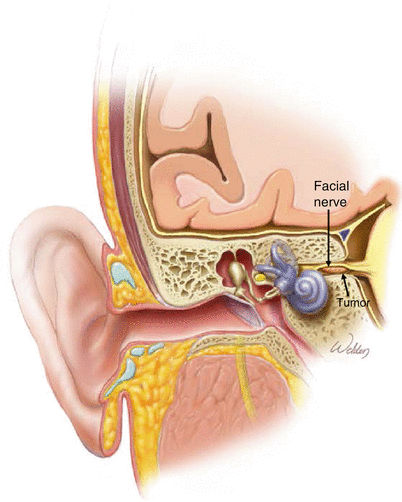

2.
Theory of the middle fossa approach to the internal auditory canal. Elevation of the middle fossa dura is performed, followed by drilling out the petrous apex to expose the posterior fossa dura and the dura of the internal auditory canal. These are then opened to expose the tumor.

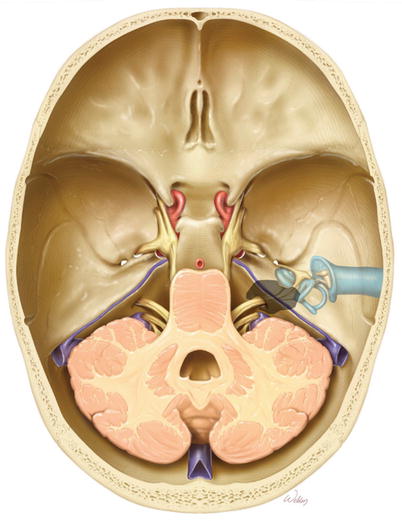
3.
Overview of the bone to be removed (gray).

4.
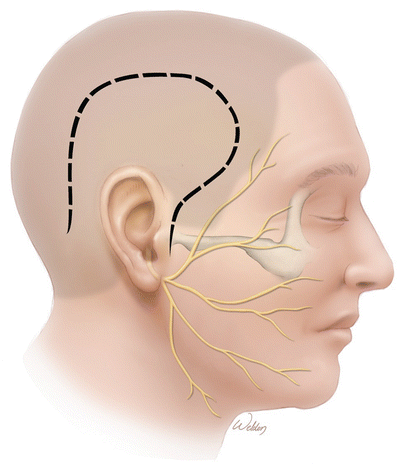
To start the procedure, an inferiorly based U-flap provides excellent exposure. The important point is to start the anterior limb down at the root of the zygoma. This is necessary to provide enough anterior–inferior exposure and provides the required line of sight to the internal auditory canal. An inadequate opening may seriously impair the ability to expose and remove the tumor. It is a critical step in the operation.
This incision can be shortened if desired. It still needs to start at the root of the zygoma, but the posterior limb may be reduced in length as long as adequate retraction is possible. Although it is possible to not clip any hair, we do remove some just along the incision line to facilitate closure. Cosmetically, this is camouflaged by the surrounding hair.

5.
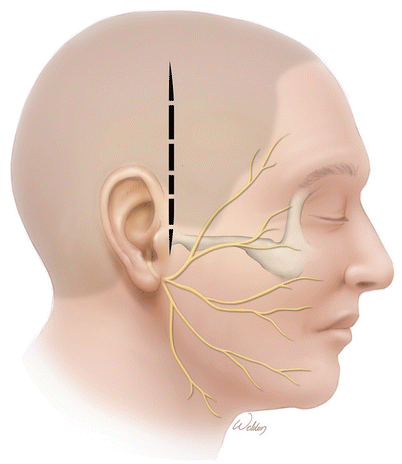
An alternate incision is vertical, which splits the fibers of the temporalis muscle. Although the ultimate opening is a little smaller than the U-flap, there is often less postoperative atrophy of the temporalis muscle. The incision choice should be influenced by the patient’s scalp and temporalis muscle thickness. This incision can be limiting, particularly in the anterior inferior corner in patients with thick scalps and a big temporalis muscle.

6.
The skin, temporoparietal fascia, temporalis muscle, and periosteum are elevated off the skull. These tissues can be elevated as a single, thick flap rather than dissecting between each layer. This reduces the risk of a postoperative hematoma forming between the layers and is more efficient.
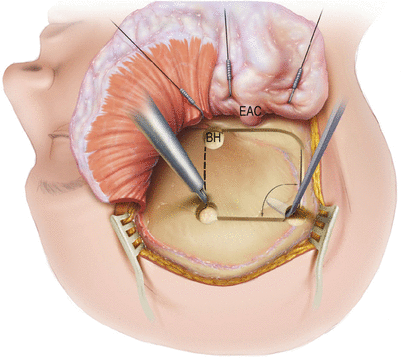
A burr hole (BH) is first placed just anterosuperior to the root of the zygoma. The next two burr holes are drilled about 4 cm superiorly and are centered over the external auditory canal (EAC). While a posterior–inferior burr hole can also be made, it is not necessary.
The dura is separated from the underside of the bone flap using an elevator. Care is taken to minimize this dissection in regions outside the bone flap as this raises the risk of a post-op epidural hematoma.
Finally, a craniotome is used to turn the bone flap. The flap is placed in moist gauze or soaked in Bacitracin solution and saved on the back table until closing at the end of the procedure.

7.
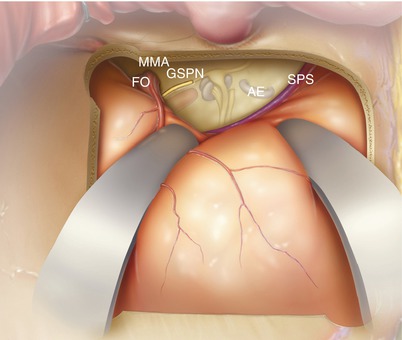
The dura is elevated off the temporal floor. This can be done with a Freer elevator, although we prefer the Joseph (narrow mastoid) elevator as it is stiffer. A key part of this portion of the procedure is to identify the greater superficial petrosal nerve (GSPN) as it leaves the temporal bone. It is often quite adherent to the overlying dura. Its fibrous adhesions to the dura can be separated with the elevator or an 11 blade scalpel. Dissection from posterior to anterior decreases the risk of injuring an exposed geniculate ganglion and dissecting under the GSPN nerve fibers.
In most cases, it is not necessary to distinguish the foramen spinosum containing the middle meningeal artery (MMA) from the foramen ovale (FO). All of this tissue contains thin-walled veins, and the more they are dissected, the more they bleed. However, for larger tumors or if a wider opening is needed, division of the MMA artery does permit more exposure of the posterior fossa. In any case, expect dissection in this area to bleed.
Once the dura can no longer be elevated anteriorly any further because of the foramen ovale, the bleeding should be controlled with hemostatic agents (such as Flowseal and Surgicel). A cottonoid should then be placed on top of it and left in place for the remainder of the case. By the time surgery is done, the cottonoid can be removed, and there is usually no further bleeding.
The elevation of the dura needs to continue medially all the way to the petrous ridge, where the superior petrosal sinus (SPS) runs. It is often easiest to identify this structure posteriorly near the junction of the transverse sinus and sigmoid sinus. This area also tends to bleed and can be controlled with hemostatic agents and a cottonoid. The SPS can then be followed as it courses anteriorly and medially. This is helpful in identifying the true posterior/medial edge of the petrous bone as there are often misleading false ridges.
The arcuate eminence (AE) is a bulge that approximates the location of the superior semicircular canal.
Dural elevation begins posteriorly, and the petrous ridge and superior petrosal sinus are identified first. Dissecting medially and anteriorly will then permit identification of the GSPN as described. These two landmarks (the petrous ridge and the GSPN) should give you sufficient orientation to anticipate the location of all other structures. There is a tendency to not dissect far enough medially along the petrous apex, and it is also important to be certain your retractor fits over the true posterior ridge of the petrous bone.
A modified version of the House-Urban and Fisch middle fossa retractors can be used instead of malleable retractors held by the Mayfield head holder if desired. The main benefit of these middle fossa retractors is that a Mayfield head holder is not needed as it is securely fixed to the edges of the craniotomy. Furthermore, the modified version has the benefit of a wider base to accommodate larger craniotomies while still lowering the profile of the blade by curving it. Both of these retractors permit the generation of significant pressure to hold the dura. The downside of these retractors is that they are finicky and take a lot of time to orient and reorient it as the dissection progresses. They don’t offer as much adjustability and retraction ability as the malleable retractors either.

8.
To reach the internal auditory canal and posterior fossa, the petrous bone needs to be drilled away. The retractors are positioned so that their tips are held securely in place at the petrous ridge. Care is taken to bend the retractors (bend) so that the lateral temporal lobe is not overly compressed. The dark dotted line highlights the area of bone to be removed deeply, and the light dotted line highlights the area of bone to be removed in a more shallow fashion so as not to injure the geniculate ganglion, cochlea, or superior semicircular canal. Nevertheless, bone in this region does need to be removed in order to visualize the deeper regions of the drilled-out cavity, and fear of damaging hearing or the facial nerve should not prevent one from obtaining enough exposure to adequately perform the procedure.
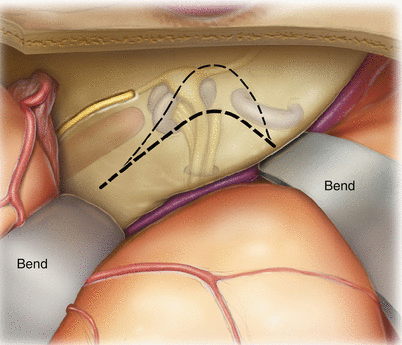

9.
After removal of the petrous bone deep to the superior petrosal sinus (SPS), the posterior fossa dura is visible (asterisks), and the internal auditory canal has been skeletonized. Note the >180° exposure of the internal auditory canal.
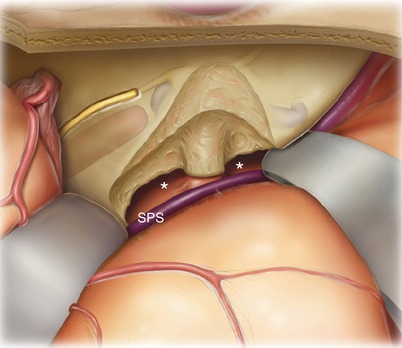

10.
The complete view depicted in the illustrations is not obtainable in surgery without rotating the microscope to the three different angles depicted. The retractors do not need to be adjusted, just the microscope.
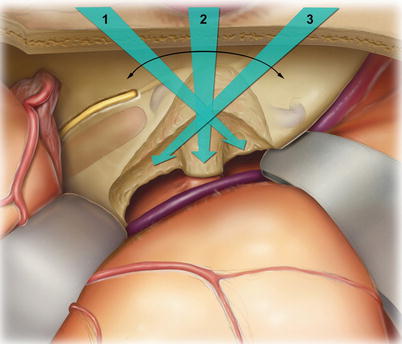

11.
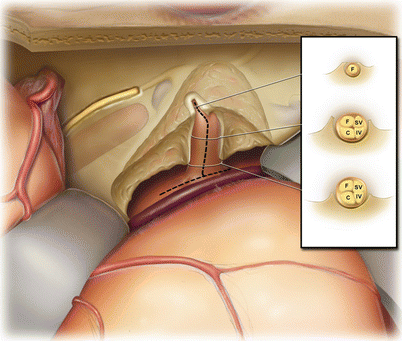
Appropriate decompression of the internal auditory canal is essential for a good outcome. The two most difficult and important areas to be dissected are the lateral end of the canal and the bone posterior to the internal auditory canal and medial to the superior semicircular canal. The dissection should continue laterally until the labyrinthine segment of the facial nerve is decompressed and the first millimeter or so of the superior vestibular nerve exposed. The superior semicircular canal should be positively identified so that bone can be removed up to it for decompression of the posterior aspect of the internal auditory canal. This space that is created provides room to push the tumor into as it is dissected medially and anteriorly.
The inset depicts the layout of the nerves of the internal auditory canal: facial nerve (F), cochlear nerve (C), superior vestibular nerve (SV), and inferior vestibular nerve (IV). The degree of drilling necessary along the internal auditory canal varies. Deeper troughs are needed medially at the porus acusticus (inset, bottom panel) than medially (inset, middle panel). Along the labyrinthine portion of the facial nerve, only a very shallow exposure is performed (inset, top panel). The cochlea is less than 1 mm away. Watch carefully when drilling at the fundus and along the labyrinthine facial nerve. The cochlea can be “blue lined” much like the superior canal without causing injury. If it is accidentally opened, do not suction out the intracochlear fluids. Gently, seal the opening with bone wax.
The dotted line indicates the dural opening. This is performed along the posterior fossa dura with an 11 blade scalpel or microscissors. Then, the nerve stimulator is used to identify the rough location of the facial nerve within the internal auditory canal by stimulating through the dura. An angled sickle knife is then used to open the dura of the internal auditory canal from lateral to medial in an area away from the nerve. The dura becomes flimsy at the fundus and easily tears open.

12.
After opening the dura, the facial nerve is typically found on top of the tumor. In this case, the tumor is depicted as an inferior vestibular nerve tumor. The facial nerve is usually splayed somewhat and covers part of the tumor. It is important to zoom in with the microscope and visually identify the posterior edge of the facial nerve (arrows). The nerve stimulator should also be used to confirm this critical landmark. This plane can be developed sharply with a #1 or #2 Rhoton dissector or similar preferred instrument.
Then, a window can be cut into the tumor using microscissors (dotted lines). This should not be directly adjacent to the facial nerve but a little bit posterior to its edge. This is so that the interface between the facial nerve and the tumor capsule is not lost.
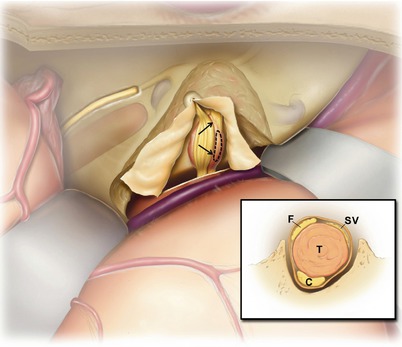

13.
For a tumor extending into the cerebellopontine angle, a similar procedure is performed, but the window can be larger. The core of the tumor is then debulked with a small cup forceps and microscissors through the window (not shown). This releases the tension from the facial nerve, reduces its splaying, and makes the nerve even easier to visualize.
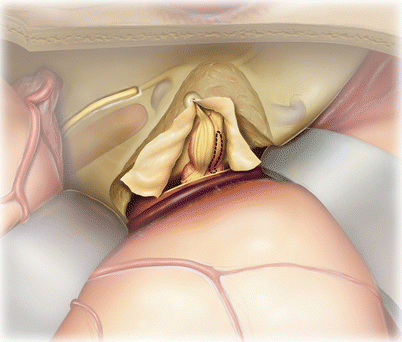

14.
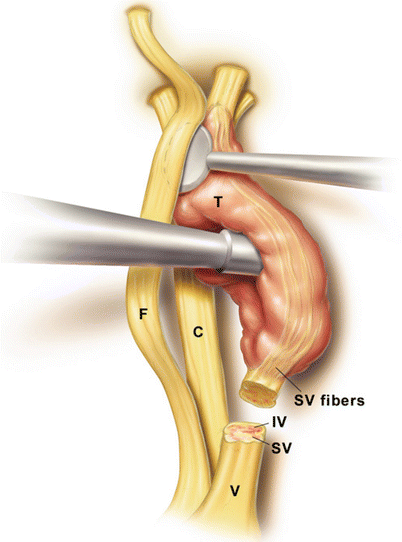
The proximal vestibular nerve (V) is transected with a microscissors. This includes both the inferior vestibular (IV) and superior vestibular (SV) nerves. Careful microdissection is then performed to separate the tumor (T) off the facial nerve (F). Sharp dissection is often helpful for this process. Dissecting medially to laterally, when possible, is helpful as it reduces tension on the facial nerve as it enters the temporal bone.
The auditory nerve (also known as the cochlear nerve, C) is under the tumor and difficult to see until this point. If the tumor is not infiltrating into the auditory nerve, there is typically a plane between the tumor and the auditory nerve which clearly presents itself. This allows the surgeon to lift the tumor off the auditory nerve in order to spare hearing. The labyrinthine artery may also be noted (not shown) but is difficult to spare if adherent to the tumor.
If they are to occur, changes in the ABR usually occur during this point in the process. Changes may occur even if the auditory nerve is spared. This indicates that there was trauma to the labyrinthine artery (spasm or division) or to the auditory nerve (edema or division). In any case, there is little the surgeon can do to restore the ABR once it is lost. Similarly, if the tumor infiltrates the auditory nerve or labyrinthine artery, there is no way to preserve hearing. These issues reflect properties of the patient’s tumor, not the surgeon’s technical skills. Tumors that extend fully to the fundus (and particularly if they extend under the transverse crest and into the base of the modiolus) are much more difficult to remove completely with hearing preservation. Soft tumors will fragment and small residuals may be left behind; removal of firm tumors may result in stretch injury to the nerve fibers or artery as the tumor is pulled away.
Finally, the distal superior and inferior vestibular nerves are transected and the tumor removed (not shown).

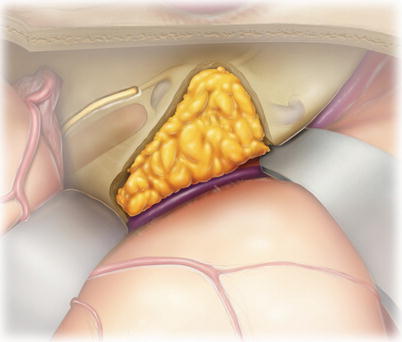
15.
Exposed air cells within the area of petrous bone drilling are plugged with bone wax (not shown). Often, this works best by applying a round piece of bone wax stuck to the back of a thin elevator or dissector. A bipolar forceps is then used to push a cottonoid into the bone wax, compressing the wax into the air cells.
Finally, a piece of abdominal fat is placed into the bony defect. While a muscle plug can be used, it tends to become highly vascularized and enhance with gadolinium within a few months. This can make it difficult to distinguish between the muscle plug and tumor recurrence by MRI. On the other hand, when a fat graft is used, fat saturation MRI techniques can eliminate this potential for artifacts.

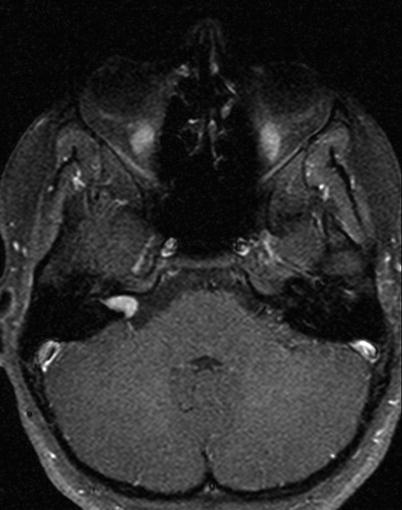
16.
Case 1: Gadolinium-enhanced T1 MRI axial section of an intracanalicular left vestibular schwannoma (arrow). This tumor would be ideal for removal by the middle fossa approach.
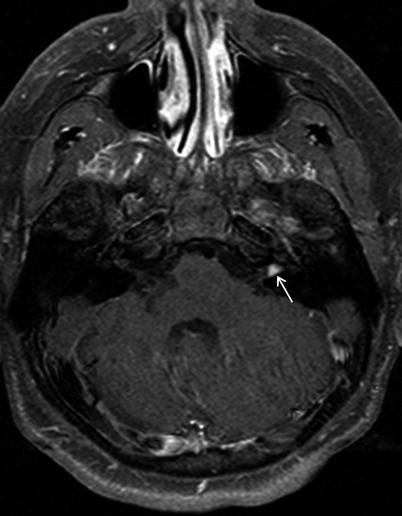

17.
FIESTA T2-weighted sequence of the same tumor. The CSF space lateral to the tumor (between the tumor and the fundus) increases the chances of hearing preservation.
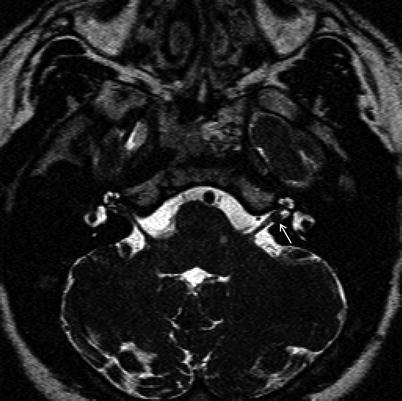

18.
Case 2: A right intracanalicular vestibular schwannoma appropriate for the middle fossa approach.