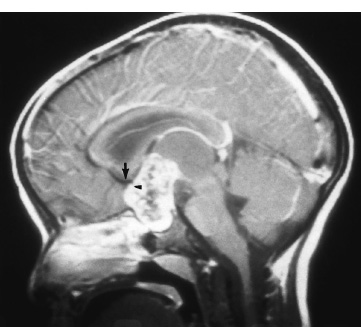
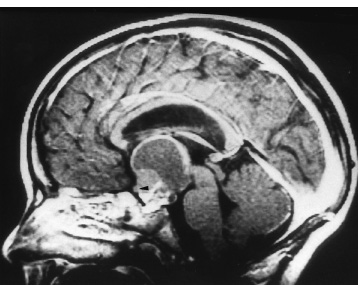
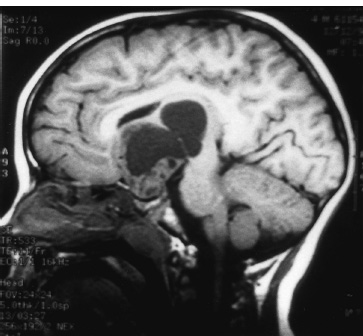
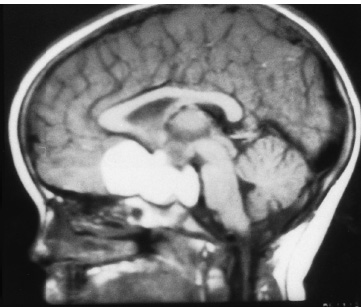
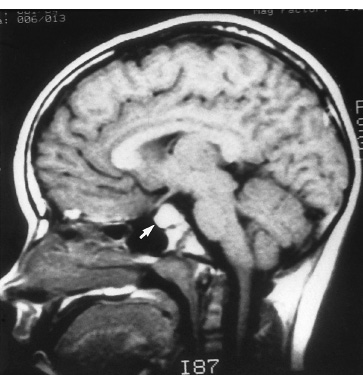
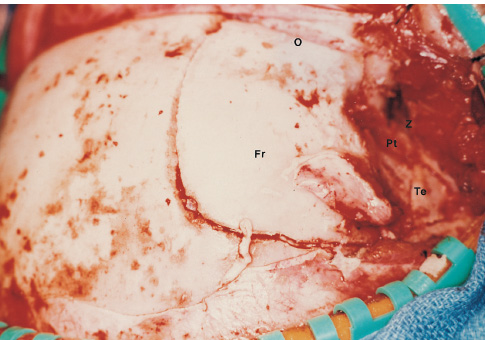
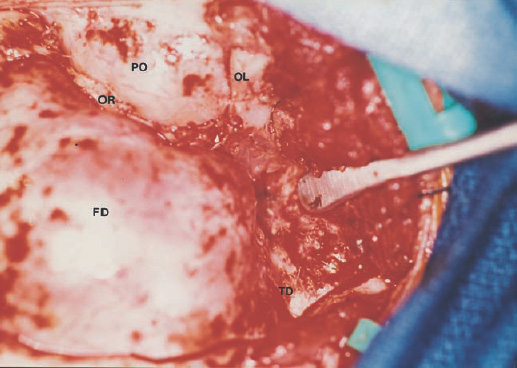
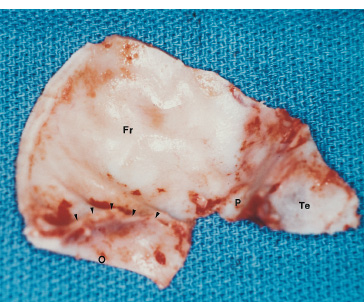
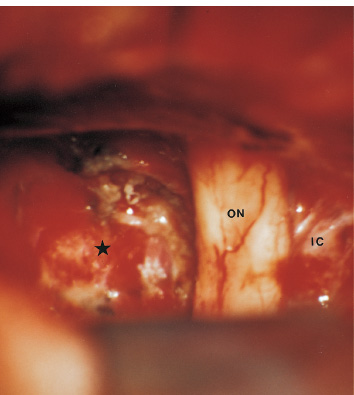
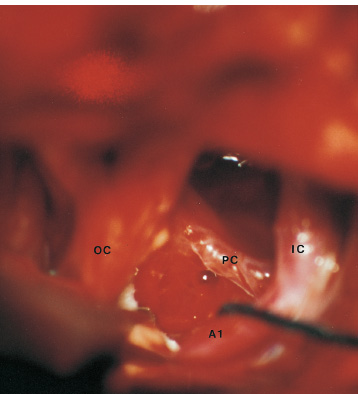
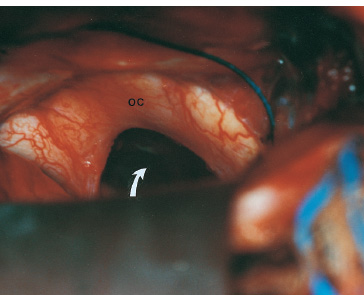
The third ventricle is a common location for pediatric brain tumors. In this location, various types of tumors can occur, and the histological distribution differs between the anterior and posterior portions of the third ventricle. Chiasmal–hypothalamic astrocytomas and craniopharyngiomas are the main tumors in the anterior third ventricle of children, whereas germ cell tumors and pineal parenchymal tumors dominate in the posterior third ventricle of children. Differential diagnosis of tumor histology and advanced neuroimaging studies are important in selecting an appropriate treatment plan. Surgical resection of tumors located in the midline is often challenging; thus, selecting an appropriate surgical approach and technique is critical. This chapter focuses on craniopharyngioma and pineal region tumors. Craniopharyngiomas originate in or above the sella turcica, and the cystic components extend in various directions: the third ventricle superiorly, the posterior fossa posteriorly, under the frontal lobe anteriorly, and into the middle cranial fossa laterally. In childhood craniopharyngiomas, calcifications and cyst formations are almost invariably present. Children often present with visual disturbances, endocrine deficits, and hydrocephalus. Hydrocephalus is the result of the suprasellar cyst, which often penetrates through the floor of the third ventricle and fills the third ventricle. Because the cyst wall is composed of neoplastic tissue, residuals of not only the solid portion but the cyst wall as well will guarantee recurrence if tumor resection is incomplete; however, as a result of the proximity of the optic pathway, hypothala-mopituitary axis, and the circle of Willis, total resection is difficult and often is complicated by postoperative neurologic and endocrine deficits. Therefore, some prefer limited resection followed by radiation therapy (RT); however, I recommend at least an attempt at complete resection within a safe margin because of the uncertain responsiveness of craniopharyngiomas to RT and concerns of latent radiation toxicity in children. Both computed tomography (CT) and magnetic resonance imaging (MRI) are routinely obtained preoperatively. For the disclosure of calcification, CT is superior to MRI; however, detailed information in regard to multiplicity of cysts and their extension are better appreciated by multiplanar MRI images. Multiple noncommunicating cysts are shown in a different intensity on MRI. Angiography is seldom necessary. Noninvasive magnetic resonance angiography provides sufficient information in regard to the relationship with the arterial system of the circle of Willis. The location of the solid tumor, the extension of any cyst and its relationship with carotid arteries, anterior cerebral arteries, and the basilar artery should be noted on neuroimaging. Careful review of the MRI scan may disclose the position of the optic nerve. On sagittal MR images, the chiasm and the anterior communicating artery are elevated in the case of a postfixed chiasm (Fig. 12–1), whereas they are located just above the tubercu-lum sella in a case of prefixed chiasm (Fig. 12–2). As stated, the extension or presence of the cyst in the third ventricle tends to cause hydrocephalus. Craniopharyngiomas of this type already have broken through the floor of the third-ventricle, directly exposing their capsule in the third ventricle cavity (Fig. 12–3). On the other hand, some children with craniopharyngiomas may have an intact floor of the third ventricle (Fig. 12–4). It is important to use preoperative neuroimaging to distinguish these two groups; both a interhemispheric transcallosal approach and a lamina terminalis approach would traumatize the floor of the third ventricle in the later group. FIGURE 12–2. Midsagittal MRI showing a cystic craniopharyngioma in the suprasellar and third-ventricle location. Note that the optic chiasm (arrowhead) is depressed (prefixed chiasm). FIGURE 12–3. Midsagittal MRI showing a massive craniopharyngioma extending into the third ventricle from the sellar region through the disrupted floor of the third ventricle. Ophthalmologic examinations should be conducted carefully to identify visual deficits and should include ophthalmoscopic examination to disclose the presence of optic atrophy or papilledema. Pupillary responses to light, particularly a swinging flashlight test, discloses afferent deficits, if any are present. Also, formal tests for visual acuity and field need to be done preoperatively. FIGURE 12–4. Midsagittal MRI showing a large cystic craniopharyngioma lifting the third-ventricle floor with an extension to the subfrontal region and the posterior fossa. Once blood samples are drawn for endocrine study, corticosteroid hormone therapy is initiated. The usual stress dose of hydrocortisone is 30 mg/m2 per day, and the maintenance dose is 5 to 10 mg/m2 per day; however, most children with craniopharyngioma present with relatively acute symptoms resulting from optic nerve compression or hydrocephalus. In these circumstances, they usually receive a high dose of dexamethasone, 2 to 10 mg every 6 hours, depending on patient size and the severity of the presenting symptoms. If hypothyroidism is noted, thyroid hormone needs to be replaced. First, the goal of surgery is determined: biopsy alone, cyst drainage alone, or radical tumor resection. Biopsy or cyst drainage can be attained by stereotaxic or ventriculoscopic procedures. If major cytoreduction and decompression are the goals of surgery, however, the optimal approach to intrasellar, suprasellar, and third ventricular craniopharyngiomas must be selected. Intrasellar craniopharyngiomas (Fig. 12–5), although rare in my experience (only 3 of 40 childhood craniopharyngiomas), are best approached transsphenoidally. Most craniopharyngiomas are accessible through a pterional–subfrontal approach, but in special cases, such as primarily third-ventricular craniopharyngioma, the interhemispheric transcallosal approach is used. An important consideration at resection of the craniopharyngioma is to attain a relaxed brain and a better angle to the sellar and suprasellar region. Hydrocephalus associated with craniopharyngioma may be favorable for the operating surgeon because a relaxed brain is achieved by intraoperative decompression of the ventricle and provides effortless brain retraction during skull-base manipulation. The presence of hydrocephalus makes it easier to manipulate the third ventricular mass through an enlarged foramen of Monro when the transventricular route is used. FIGURE 12–5. Midsagittal MRI showing an intrasellar craniopharyngioma (arrow). A transsphenoidal approach is suitable for tumor resection. A burr hole is made just anterior to the coronal suture on the midpupillary line of the right side. The dura is coagulated and incised. Ventricular puncture is done by aiming toward the nasion in the coronal plane and the tragus of the ear in the sagittal plane. In older children, the distance from the outer table of the skull to the foramen of Monro is 6.5 cm. When the tumor cyst is occupying the third ventricle, ventriculoscopy is used to inspect the ventricular space. The cyst walls often show yellow–green discoloration. If feasible, I would puncture the cyst to drain the cystic content through a ventricular catheter under ventriculoscopic control. In most cases of craniopharyngioma, I use a combination of pterional and subfrontal approaches from the right side. For this approach, the patient is orally intubated and placed in a supine position. A three-pin head fixation device is used for older children, but a horseshoe headrest is used for infants and younger children with a thin skull. The head is rotated toward the left side about 15 degrees, and the neck is extended slightly backward. The scalp incision is made in a bicoronal fashion anteriorly and superiorly to the tragus of the right side and extending along the coronal suture to the superior temporal line of the left side. The skin flap is turned subperiosteally. The right temporal muscle is sectioned vertically up to the zygomatic process by using cutting cautery. Anteriorly, the superior temporal line is cut, and the temporal muscle is turned with the scalp flap. At the orbital ridge, the superior orbital foramen and nerve are identified. The caudal portion of the supraorbital foramen is chiseled away, and the superior orbital nerve is turned forward with the scalp flap without sacrificing it. The pericranium is sharply dissected at the superior orbital ridge, and the periorbita is separated from the roof of the orbit. The scalp flap needs to be turned forward sufficiently to expose the glabella. For a fronto-temporo-orbital craniotomy, four burr holes are made. One is made directly on the pterion, and is enlarged anteriorly and posteriorly to expose the frontal and temporal dura. Then the pterion is removed caudally as much as possible. Another burr hole is made at the keyhole (frontozygomatic junction), and both the frontal base dura and periorbita are exposed. The third burr hole is made on the glabella, and the fourth burr hole is made parasagittally in the posterior frontal bone of the right side. The fronto-temporo-orbital craniotomy is performed as follows: Craniotomy is made using a cran-iotomy from the temporal burr behind the pterion to the posterior frontal burr hole and then to the burr hole at the glabella. The burr holes at the pterion and the keyhole are connected (Fig. 12–6). Subsequently, an orbitotomy is carried out between the key hole and glabella burr holes. A Gigli guide is placed through these burr holes in the anterior cranial fossa, allowing placement of the Gigli saw. By using the Gigli saw on the orbital roof from the anterior cranial fossa to the orbit, the orbitotomy is completed (Fig. 12–7). During that time, the orbital contents need to be protected by a spatula, and the orbitotomy is made as posterior as possible. A free bone flap, which includes the frontal, temporal, and orbital rims and the anterior orbital roof, is attained (Fig. 12–8). Once the craniotomy is completed, the sphenoid ridge is removed epidurally from the pterion as medially as possible. The dura is incised along the frontal base extending over the Sylvian fissure to the temporal region. Once the dura is open, the posterior inferior frontal lobe is elevated along the sphenoid wing. Further medially, the anterior clinoid process and, subsequently, the planum sphenoidale are identified. Posteriorly to the planum sphenoidale is the arachnoid of the suprasellar cistern. Once the cistern is opened and the cerebrospinal fluid (CSF) is drained, the relaxed brain allows further elevation of the frontal lobe with gentle retraction. Then the medial sylvian fissure is opened. Once the suprasellar and sylvian cisterns are open, the right and left optic nerves, the optic chiasm, and the right internal carotid artery are in the surgical view. The carotid artery is present lateral and inferior to the right optic nerve. Further elevation of the gyrus rectus of the right side allows for identification of the lamina terminalis and the A1 segment of the right anterior cerebral artery and anterior communicating artery posteriorly. FIGURE 12–6. Right fronto-temporo-orbital craniotomy is shown. O, orbital ridge; Pt, pterion; Z, zygomatic arch; Fr, frontal bone; Te, temporal bone. FIGURE 12–8. Inner surface of a free bone flap of the fronto-temporo-orbital craniotomy is shown. It includes the orbital roof (arrowheads indicate the posterior edge of the orbital roof). Fr, frontal bone; Te, temporal bone; P, pterion; O, superior orbital ridge. Neural and vascular structures are inspected under microscopic magnification once the chiasmal cistern is exposed. The nature of the optic chiasm determines whether it is fixed posteriorly (postfixed) or anteriorly (prefixed). In the case of postfixed chiasm, the craniopharyngioma capsule is present in the prechiasmatic space (Fig. 12–9). This enlarged prechiasmal space becomes the primary access to the tumor. Also, the space between the right optic nerve and the carotid artery (opticocarotid space) is an important access site for the tumor resection (Fig. 12–10). In a case of prefixed chiasm, the prechiasmal space is virtually absent; therefore, the lamina terminalis is opened, and the tumor is removed through it. The lamina terminalis is somewhat pale, bulgy, and less vascular. One should take care not to traumatize the anterior communicating artery, which is located just above the lamina terminalis. Any intrasellar mass can be removed subsequently by reducing the retrochiasmal mass, which often creates an enlarged prechiasmal space. The widened opticocarotid space of the ipsilateral side provides an important access site to the tumor under the chiasm (Fig. 12–11). Tumor under the chiasm, or deep in the anterior sella turcica, is often difficult to remove when the prechiasmatic space is absent. The right anterior clinoid process is removed through an epidural approach, which, once removed, gives further access to these regions. FIGURE 12–10. Following resection of the prechiasmal craniopharyngioma, both optic nerves and the left carotid artery are shown. The opticocarotid space is shown next to the right optic nerve (arrow). FIGURE 12–11. The optic nerve and chiasm (OC) is displaced, and the right opticocarotid space is widened. Note the large access to the region under the optic chiasm. The posterior communicating artery (PC) is visualized, together with the internal carotid artery (IC) and anterior cerebral artery (A1). Once the craniopharyngioma capsule is opened, internal decompression is achieved, and the cystic contents are aspirated. The solid portion, which consists of brittle, calcified tissue, is removed in a piecemeal fashion. Some areas are densely calcified, and piecemeal removal may be difficult. Forcible removal of large, calcified tissue through the prechiasmal space or retrochiasmal space will traumatize the highly sensitive optic structures. I use the carbon dioxide laser to dehydrate a highly calcified tumor and then break it into small pieces for removal. It is important to debulk the intracapsular content as much as possible. The cystic contents of a third ventricular cyst can be drained once the solid component in the suprasellar area is reduced. Once sufficient internal decompression is achieved, the tumor capsule is gently and meticulously separated from the optic and vascular structures. In a case of post-fixed chiasm, the capsule is separated from the ventral surface of the chiasm. The right optic nerve is also separated away from the tumor at its ventral surface. The internal carotid arteries are always separable from the craniopharyngioma at primary resection. The tumor capsule is gently separated from the carotid artery through the interoptic space and optic carotid space. Laterally and inferiorly to the carotid artery is the right third nerve, which should be identified. A further opening of the sylvian fissure facilitates tumor separation from the third nerve. The left optic nerve moves into the surgical field once the internal decompression is achieved and is carefully separated. The left internal carotid artery is identified by gentle retraction of the tumor to the right side and is separated from the tumor capsule. The tumor capsule is coagulated by bipolar cautery, which not only reduces the size of the capsule and tumor but also makes the capsule firmer. The tumor capsule is gently retracted continually while separating it from the surrounding structures. The pituitary stalk is usually present behind the solid portion and may be displaced in either direction. The appearance of the pituitary stalk has a distinctive reddish color and has vertically oriented blood vessels along its course; however, it may be totally replaced by the tumor. Although preservation of the pituitary stalk is ideal, in most cases this is difficult because of its involvement by the tumor. If the pituitary stalk needs to be sectioned, this should be done in the most distal portion. Although the ventral chiasmal surface is easily separated from the tumor, the tuber cinereum is always adherent to or replaced by the tumor. The trajectory of the microscope needs to be changed to inspect the ventral surface of the chiasm. The fronto-temporo-orbital craniotomy provides a better angle for that purpose. Continuous gentle retraction of the capsule brings the tuber cinereum into the surgeon’s view. Under direct vision, the tumor capsule is separated from the tuber cinereum. It is important not to break the continuity of the capsule to attain complete removal of the craniopharyngioma. If the capsule breaks during the retraction, that portion of the capsule will migrate back into the third ventricle and become difficult to resect. Craniopharyngiomas, which extend posteriorly and compress the brainstem, can be removed by gentle retraction because the basilar artery and the brainstem are separated from the tumor capsule by the Lillequist membrane. In a case of prefixed chiasm, the primary route for the resection is though the lamina terminalis (Fig. 12–12). One should confirm that the tumor is present directly in the third ventricle following internal decompression. The tumor resection is carried out as already described. The portions of the tumor from the third ventricle and the retrochiasmal region are removed with relative ease, but resecting the tumor under the chiasm and the sella turcica is often difficult. Following removal of craniopharyngioma from the third ventricle, the prechiasmal space may be widened; however, often this space is not enough for tumor resection from these areas. The opticocarotid space needs to be used in such a case. If this does not suffice, I would resect the anterior clinoid process extradurally and the lateral portion of the planum sphenoidale of the right side by means of a high-speed drill, which exposes the proximal portion of the optic nerve, thus creating the prechiasmal space intradurally.
CRANIOPHARYNGIOMA
Surgical Indications and Preoperative Evaluation
Preoperative Management
Operative Planning
Intraoperative Technique: Ventriculostomy
Positioning
Initial Exposure
Craniotomy
Dural Opening and Tumor Exposure
Tumor Resection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


