(1)
University South Florida, Tampa, FL, USA
Abstract
Many factors are involved in the surgical management of adult spinal deformity, including maintenance of coronal and sagittal balance as well as spinopelvic harmony [1–5]. Adult spinal deformity (ASD) is believed to develop because of asymmetrical degeneration of discs, osteoporosis, and vertebral body compression fractures [6]. Presenting symptoms of this condition primarily include radiculopathy, chronic low back pain, and neurogenic claudication caused by concurrent spinal stenosis [7, 8].
28.1 Introduction
Many factors are involved in the surgical management of adult spinal deformity, including maintenance of coronal and sagittal balance as well as spinopelvic harmony [1–5]. Adult spinal deformity (ASD) is believed to develop because of asymmetrical degeneration of discs, osteoporosis, and vertebral body compression fractures [6]. Presenting symptoms of this condition primarily include radiculopathy, chronic low back pain, and neurogenic claudication caused by concurrent spinal stenosis [7, 8].
Studies by Schwab et al. [9, 10], Glassman et al. [11, 12], and others [13] have demonstrated that in the treatment of congenital and acquired deformity, correction of sagittal alignment to an SVA (sagittal vertical axis) < 5 cm leads to improved HRQOL outcomes. With a positive sagittal alignment, there is increased stress on the axial musculature, which in turn leads to abnormal degenerative changes in the disc spaces, resulting in further imbalance and serving a self-perpetuating cycle. A high postoperative SVA also increases the risk for pseudarthrosis, adjacent segment disease, and proximal functional kyphosis [14–16]. Although currently no practice guidelines exist regarding MIS sagittal deformity correction, in addition to the SVA, here are a few key questions that should be addressed during the treatment decision-making process:
3.
Is the pelvic tilt < 25°?
4.
How many degrees of sagittal correction is the goal?
5.
Are MIS techniques feasible or would traditional open procedures be indicated?
Despite the many benefits of minimally invasive surgery (MIS), one of the limitations of MIS techniques is that up until now they have been unable to improve sagittal balance significantly [19]. Sagittal imbalance is traditionally managed with posterior shortening osteotomies, anterior lengthening maneuvers, or both. Classically, closing wedge osteotomies include a Smith-Petersen osteotomy (SPO), a pedicle subtraction osteotomy (PSO), or a vertebral column resection (VCR), which have been reported to have a 41 % complication rate in ASD [20, 21]. Major complications in revision adult deformity surgery were reported by Cho et al. to be 34 % in a retrospective review of 141 patients [22].
For these reasons, alternative minimally invasive techniques with reduced morbidity are being proposed for improving sagittal balance in ASD. A few examples of this cutting-edge technology that will be explored in this chapter include:
Also, less invasive facetectomy in conjunction with the prior techniques can potentially provide additional lordosis when compression is applied to the construct having the interbody implant as a pivot. In addition, a recent report demonstrates that significant sagittal correction can be achieved using multilevel MIS transforaminal lumbar interbody fusions (TLIF) in conjunction with percutaneous pedicle screw fixation, which in itself is a hybrid open-MIS technique that will need to be explored further [25]. Up to this point, literature on these techniques has been scarce, and most reports are scattered with no homogeneity or agreement on techniques. We will attempt here to systematically describe several MIS options in the treatment of sagittal imbalance, as well as the anatomy encountered from the lateral approach.
28.2 Patient Selection
In addition to technological advancements in spine instrumentation that allow us to treat different pathologies more efficiently, our understanding of the optimal relationship between the spine and pelvis has evolved. We now understand that if certain spinopelvic parameters are realized during surgical management, patient satisfaction and overall outcomes are improved, as well as a potential reduction in adjacent segment disease. The goals often cited are:
1.
Sagittal vertical axis < 5 cm
2.
Pelvic incidence within 9° of lumbar lordosis
3.
Pelvic tilt < 25°
In the not-so-distant past, it was adequate to work up a patient with intractable low back pain with a lumbar x-ray only. However, given what we now know about spinopelvic harmony, it almost seems an injustice to our patients to not obtain standing spine x-rays with a 36″ cassette. Although at this point the majority of spine surgeons are not analyzing sagittal balance and pelvic parameters, it seems that national spine organizations for both neurosurgery and orthopedic surgery are encouraging their members to embrace these new philosophies. Hopefully in the near future, 3′ standing x-rays of the entire spine will be available to all spine surgeons and performed on all patients evaluated for surgery. It must be realized that it can be dangerous to treat all patients the same and that each patient must be treated on an individual basis. However, much like with minimally invasive surgery, each patient should be viewed with a “new eye,” with the goal of safely attaining spinopelvic harmony in the most minimalistic manner possible.
28.3 Advantages and Disadvantages
As discussed previously, open surgical techniques for the correction of sagittal imbalance include posterior shortening osteotomies/VCR and anterior release. With anterior release, historically there has been significant approach-related morbidity, limiting its utility [26]. Posterior osteotomies are also limited by long operative times, heavy blood loss, significant postoperative pain, and possible neurologic compromise related to the manipulation of neural elements. However, even given the significant morbidity associated with a PSO, it is currently the most effective means of improving sagittal balance, assuming a 30° correction per level of PSO. It also enables the surgeon to provide direct neural decompression. Though less evidence exists regarding MIS ALL release, recent literature suggests that through the lateral transpsoas approach, we are able to achieve 10–15° of sagittal correction per level of ALL released, with much shorter operative times and reduced morbidity [23, 27, 28]. However, we must be aware that further study is necessary to be able to reliably reproduce MIS techniques for the correction of sagittal imbalance.
28.4 Anterior Longitudinal Ligament Section via the Lateral Transpsoas Approach
Recent advances in minimally invasive spine surgery allow for options in the treatment of spinal deformity, as many new techniques may offer similar or even improved clinical and radiographic outcomes with less morbidity than conventional approaches [29]. In particular, the lateral retroperitoneal or retropleural approach allows access to the spine with minimal tissue disruption along a relatively bloodless plane and has been shown to preserve coronal and sagittal balance [30, 31]. However, in cases of fixed sagittal imbalance, ALL release (anterior column release-ACR) may be useful to restore proper alignment, as it can potentially provide sagittal correction similar to an SPO based on emerging data [23, 27, 32]. This requires further investigation as the technique becomes more utilized.
As with any operation, meticulous preoperative planning and identification of appropriate candidates for ACR is essential. We realize that this is an evolving technique still with much left to discover; however, we hope that in describing our method of performing an ACR, surgeons may be encouraged to attempt or modify this procedure with a goal of continuous improvement.
Releasing the ALL is technically demanding and requires a thorough understanding of the regional anatomy of the lateral retroperitoneal or retropleural approach to the spine. Though it reduces some complications associated with posterior approaches, it is associated with its own unique set of risks in addition to those related to the standard MIS lateral access. As with other MIS techniques, there is a significant learning curve and this will likely foster initial skepticism given the potential complications associated with an ACR. However, the potential benefit to patients is significant and thus should be explored further.
As with preoperative evaluation for any planned lateral retroperitoneal approach, it is essential to evaluate the patient’s MRI to determine the course of the great vessels and where the bifurcation occurs [33, 34]. In addition, care must be taken not to injure the ilioinguinal, iliohypogastric, or lateral femoral cutaneous nerves when accessing the retroperitoneum [34–36].
However, given its posterior to anterior course along the iliopsoas muscle at the most frequently accessed levels (L2/3 and L3/4), the genitofemoral nerve is particularly at risk. Depending on its course, opening the retractor may cause a neuropraxia that is likely dependent on both the timing and amount of stretch of the nerve. Risk of injury to this nerve may also occur when performing anterior interspace dissection to reach the ALL [37]. Although the use of directional electrophysiologic monitoring can help minimize nerve injury during the lateral approach, the genitofemoral nerve is mainly sensory and unable to be monitored without scrotal or labial electrodes [38].
Another pitfall comes with dissecting the sympathetic plexus and great vessels off the ALL using the curved custom retractor, which carries the risk of a potentially catastrophic aortic or IVC injury. The target of the retractor blade is the plane of dissection dorsal to the sympathetic plexus/great vessels and ventral to the ALL. However, a unique problem associated with this procedure is dissection in the plane ventral to the sympathetic plexus and dorsal to the great vessels. If this occurs unbeknownst to the surgeon, inadvertent damage may be done to the sympathetic plexus unilaterally. At this point ramifications of such an injury have been unexamined and require further study. It is likely that injuries occur during these dissections to the gray and white rami communicantes, which connect the sympathetic plexus to the spinal nerve roots and generally run on the lateral aspect of the inferior third of the vertebral bodies. However, given the many overlapping connections existing between these structures, it is unlikely that injury at one or two levels will produce any significant clinical consequence.
After reaching the contralateral interspace with the retractor, a fresh 11-blade rather than monopolar electrocautery should be used to incise the ALL in order to prevent thermal injury to the sympathetic plexus and nearby vascular structures. Opening the intradiscal distractor should then produce a “fish-mouth” deformity at the disc space. If this does not occur, it is likely that the ALL is not fully cut. The variety of hyperlordotic cages (10°–30°) enables the surgeon to fit each disc space appropriately. It should be placed between the anterior and middle third of the disc space, which is anterior to the suggested position of a lumbar interbody cage placed from the lateral decubitus position [37, 39]. The purpose of this is to provide ligamentotaxis of the posterior longitudinal ligament (PLL) and indirect foraminal decompression at that level. One or two transversely oriented screws are used for the sole purpose of securing the cage and preventing ventral migration into the peritoneal cavity. The mechanical stability provided by these screws is likely negligible.
28.5 Anatomic Consideration
28.5.1 Anterior Longitudinal Ligament
The ALL is a strong band of fibers extending along the anterior aspect of the vertebrae. It widens as you move caudad along the spine and is thicker in the thoracic than the cervical or lumbar region [40–42]. In the lumbar spine, the ALL traverses the anterior aspect of all VB and disc spaces and is composed of three layers: superficial, intermediate, and deep. The superficial layer traverses four or five vertebral bodies, while the intermediate layer covers two or three vertebral bodies. The deep layer of the ALL covers only the individual vertebral bodies and attaches from one VB to the next. The ALL is thinner and wider at the level of the disc but thicker and narrower at the vertebral bodies. Also, it is more strongly adherent to the intervertebral disc than the middle of the vertebral body, making mobilization at the disc space difficult. There are oval apertures at the lateral aspect of the ligament to allow passage of vessels (Fig. 28.1) [40–42].
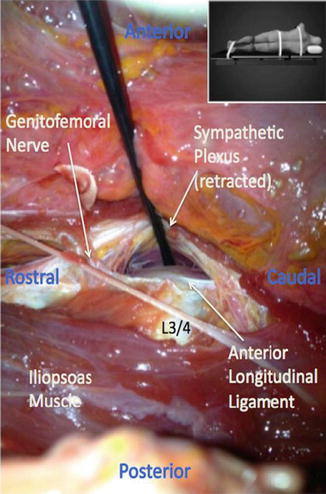

Fig. 28.1
Sympathetic plexus. Photograph during cadaveric dissection demonstrating the sympathetic plexus retracted off the anterior longitudinal ligament at the L3/4 disc space. Note the genitofemoral nerve crossing the disc space moving posterior to anterior. Inset demonstrates the position of the cadaver during dissection
28.5.2 Lumbar/Sympathetic Plexus
Neural structures at risk during this procedure are similar to those in the standard lateral approach [37, 39, 43]. However, because it courses dorsal to ventral in the psoas major, of all the lumbar plexus nerves, the genitofemoral nerve (L1, 2) is especially at risk as it crosses the L2/3 and L3/4 disc spaces. At the caudal end of the L4 VB, it has moved anterior to run along with the sympathetic plexus.
The sympathetic plexus is a paired bundle of nerves running along the anterolateral border of the vertebral bodies in the lumbar spine that functions as part of the autonomic nervous system via fibers to the inferior mesenteric ganglion [40, 41]. It lies along the lateral border of the ALL, where it meets the psoas major, and may be encountered during an ACR (Fig. 28.2). It is in communication with the lumbar plexus, with information arriving at the paravertebral ganglia through the white rami communicantes and leaving via the gray rami communicantes. These communicating fibers run along the inferolateral aspect of the VB and generally are not encountered at the disc space where an ACR is performed.
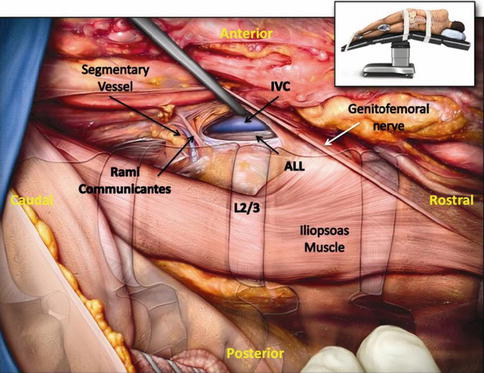

Fig. 28.2
Anterior longitudinal ligament and sympathetic plexus. Illustrated photograph during cadaveric dissection demonstrating the relationship between the anterior longitudinal ligament and the sympathetic plexus. Note the complex network of communicating nerves in connection with the sympathetic plexus. Inset demonstrates the position of the cadaver during dissection
28.5.3 Great Vessels
The aorta and the IVC lie along the left and right anterior lumbar VB border, respectively. Even when intimately associated with the ALL, there is an adipose-lined anatomic plane allowing dissection dorsal to the great vessels. There are generally four paired lumbar (segmental) arteries, which arise from the aorta and course laterally around the vertebral body, thus avoiding the disc space [41, 43–45]. [44, 45] The aorta bifurcates into the right and left common iliac arteries 18 mm rostral to the L4/5 disc space, while the right and left common iliac veins converge to form the IVC within 2 mm of the L4/5 disc space (Fig. 28.3) [33].
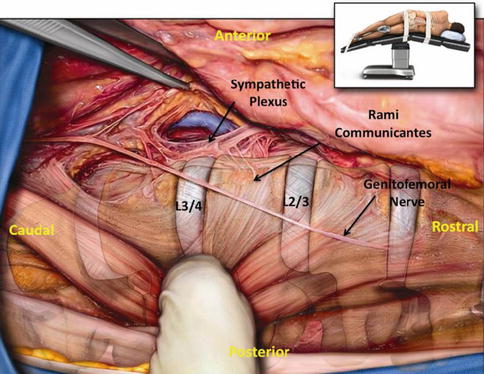

Fig. 28.3
Great vessels. Illustrated photograph during cadaveric dissection in the right lateral decubitus position (inset). Note the inferior vena cava ventral to the anterior longitudinal ligament on the contralateral side. The aorta here is being retracted. Notice also the lumbar segmental artery arising from the aorta. The sympathetic plexus is being retracted with the aorta
Retroperitoneal transpsoas approach involves traversing the nerves of the lumbar plexus both within and outside of the psoas muscle. There are four major nerves traveling outside of the psoas muscle, namely, subcostal (T12), iliohypogastric (L1), ilioinguinal (L1), and lateral femoral cutaneous (L2–L3) nerves. They originate from the posterior border of the psoas muscle and descend obliquely through the retroperitoneal space. These free nerves are most vulnerable at the initial stages of the approach during abdominal muscle and retroperitoneal dissection superficial to the psoas muscle and hence necessitate delicate blunt dissection [35]. The genitofemoral (L1–L2) nerve initially travels within the psoas muscle, across the L2/3 disc space, for a short distance before emerging and continuing on the anterior surface of the muscle.
The L2, L3, and L4 roots merge to form the femoral nerve which courses deep within the psoas muscle to pass under the inguinal ligament prior to giving off the cutaneous (medial/intermediate femoral cutaneous/infrapatellar branch/saphenous nerve) and muscular branches. Variation in proximal trajectory of the femoral nerve has been described as it traverses the psoas muscle [46, 47]. Though anatomical variations of the lumbar plexus have been described in the literature, large population studies to establish a more accurate measure of the prevalence of the surgically relevant variability are lacking [48, 49]. Variations in the trajectory of the femoral nerve should be strongly considered when establishing an operative corridor with utilization of directional EMG monitoring to prevent nerve injury. The obturator nerve (L2–L4) courses through the psoas muscle posterior to the femoral nerve. It emerges from the medial aspect of the psoas muscle and travels just lateral to the sacrum prior to exiting the pelvis though the obturator foramen. Finally, the sympathetic plexus runs on the anterior surface of the vertebral body and is at risk especially with lateral corpectomies or anterior longitudinal ligament (ALL) releases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








