Chapter 131 Motor Cortex Stimulation for Intractable Facial Pain
In the early 1990s, Tsubokawa and colleagues first reported using electrical stimulation of the primary motor cortex to alleviate post-stroke thalamic pain.1–3 The basic approach of motor cortex stimulation (MCS) is to implant an array of stimulating electrodes over the primary motor strip in the region corresponding to the territory of painful neuropathy. Chronic, intermittent electrical stimulation is then delivered at intensities below the threshold for evoking a motor response. MCS outcomes have been variable, but application of the technique to TNP has yielded success rates of 75% to 100% in many series4–20 (see Table 131-1). Despite a paucity of controlled trials, MCS has evolved into a standard therapy for disabling TNP at many centers, offering a palliative “last resort” for patients who have exhausted other avenues of treatment.
Historical Perspective
The notion that the motor cortex contributes to pain processing dates to observations made by Penfield in the first half of the 20th century. Operating on epilepsy patients, he recognized that electrical stimulation of the motor strip could elicit sensory responses.21 When a patient with chronic burning pain experienced a relapse in symptoms following a somatosensory cortex resection, Penfield ventured to resect the precentral gyrus. This yielded a complete—albeit temporary—abatement of the patient’s pain.21,22 Three of Penfield’s patients later achieved long-term relief from intractable facial pain with combined resections of the pre- and post-central gyri. Lende and Kirsch adapted Penfield’s approach in two patients with intractable face pain, removing both the precentral and postcentral areas of facial representation.23 Although apparently effective, this aggressive strategy has not been reported since.
The 1970s heralded the introduction of non-lesional techniques—notably deep brain stimulation (DBS)—for mitigating chronic pain. DBS pain targets were typically the periaqueductal or periventricular gray matter or the sensory thalamus. Success rates reported in the literature were inconsistent, and no sizeable controlled trials were conducted for decades. At present, the precise role and efficacy of DBS for chronic pain syndromes remain subjects of debate.24–26
In the early 1990s, Tsubokawa shifted the focus of pain circuit modulation toward cortical entry points. His work was motivated by animal models of deafferentation pain in which bursting hyperactivity in thalamic neurons could be inhibited by stimulation of the motor strip. In 1991, Tsubokawa and colleagues described a series of seven thalamic pain syndrome patients, each of whom realized “excellent or good” long-term pain relief with MCS.2 Subsequent series revealed that TNP patients tended to fare better with MCS than counterparts with neuropathic pain in other distributions.8,11,27
Mechanism of Action
The mechanisms underlying pain modulation by MCS remain speculative.28–31 Evidence from a range of modalities has underscored that an extensive neural circuit is dysfunctional in chronic pain syndromes.32–34 While the role of MCS in suppressing the symptoms of chronic pain syndromes is as yet unclear, experiments in both animals and humans have offered insight.
Microelectrode recordings in deafferentation animal models and humans with central pain syndromes have demonstrated that thalamic neurons manifest spontaneous bursting patterns.2,35–41 Deafferented thalami have an altered somatotopy, and microstimulation of these thalami can elicit “central allodynia”—a phenomenon in which the usual sensations evoked by stimulation are replaced by painful sensations similar to those seen clinically in pain syndromes.35,38 These lines of evidence led some to implicate thalamic hyperactivity as an engine driving the manifestations of central pain.
Concurrently, a picture was emerging that electrical stimulation of the motor cortex could suppress thalamic hyperactivity.2,36,42 At that time, there were already precedents for the hypothesis that cortical stimulation could mitigate pain. Animal studies dating to the 1950s had established that stimulation of sensorimotor cortex diminishes spinal cord afferent activity43 as well as dorsal horn activity.44 MCS also elevated nociceptive response latency45,46 and has since been shown to selectively attenuate dorsal horn responsiveness to high-intensity painful stimuli.47
Tsubokawa’s initial work was based on the hypothesis that MCS inhibits deafferented nociceptive neurons,1–339 perhaps by activating (both anti- and ortho-dromically) a mesh of superficial reciprocal fibers carrying non-nociceptive signals between the motor cortex and both adjoining somatosensory cortex and the sensory thalamus.30,32,48–52 Indeed, recent transcranial magnetic stimulation studies have suggested that chronic pain syndromes may be associated with defective intracortical inhibition, and that MCS restores this inhibition.32,51 Activation of cortical interneurons might reestablish a balance of normal non-nociceptive dominance among systems of hyperactive nociceptors, thereby effecting analgesia.29,41,53
Positron emission tomography (PET) studies permit interrogation of changes in regional cerebral blood flow (CBF) that attend MCS. Patterns of CBF redirection during (and after) MCS reflect modulation of a functional pain network that includes the anterior cingulate cortex, orbitofrontal cortex, medial thalamus, and periaqueductal and periventricular gray matter.54,55 At baseline, there is relative decrement in thalamic CBF in chronic pain patients, and this decrement is reversible with MCS.29,56,57 Analgesic effect parallels the degree of increase in anterior cingulate gyrus CBF,29,31,57–60 perhaps suggesting that MCS may blunt the affective or “suffering” component of chronic pain, a process in which this cortical region has a documented role.61 Regions hemodynamically activated by MCS retain their activation for 30 minutes or more after stimulation is halted,29,31 reminiscent of observations that MCS-associated analgesia may persist for hours after a stimulator has been turned off.3,62
Finally, some have suggested that analgesia can be ascribed to MCS-triggered neurochemical release. For example, Maarrawi et al. demonstrated that MCS enhances secretion of endogenous opioids—particularly in the anterior middle cingulate cortex and periaqueductal gray matter—in correlation with degree of analgesia.63
Indications
Importantly, the clinician should distinguish patients with TNP from those with classical trigeminal neuralgia, the latter disorder being characterized by episodic sharp, lancinating pains in a trigeminal distribution (although the taxonomy of these entities remains a point of contention64). Invasive treatments considered effective for trigeminal neuralgia may in fact intensify the symptoms of TNP. It is our practice to pursue conventional medical and neurosurgical therapies (reviewed elsewhere65–67) for trigeminal neuralgia before resorting to MCS. Of course, trigeminal neuralgia may evolve into a form of TNP. This often occurs in patients who fail neurosurgical interventions for trigeminal neuralgia; for example, rhizotomy patients may develop anesthesia dolorosa—a form of TNP. Under such circumstances, MCS represents an appropriate therapeutic option.
Outcomes
Historically, MCS has been applied to TNP more successfully than to any other neuropathic pain syndrome. One factor cited to account for this disparity is that the face is represented by a large territory of the motor strip on the accessible lateral convexity, meaning that the electrode can be placed with relative ease and consistency. Most MCS studies regard a 50% reduction in baseline visual analog scale (VAS) pain ratings as a threshold for a positive clinical response. Given this criterion, literature reviews cite composite TNP long-term response rates of 60% to 80%.4,68–71 A summary of individual MCS case series (with the number of TNP patients in each) is shown in Table 131-1.
While reports of patient follow-up beyond 2 to 3 years are uncommon, many investigators have noted that the clinical benefit of MCS may decline slowly with time, perhaps due to remodeling of cortical architecture. Anecdotal reports have suggested that a washout or “no-stimulation” period followed by stimulator reprogramming may help to regain lost clinical milestones. Henderson et al. found that intensive reprogramming could indeed yield clinical improvement; potential benefit, however, should be weighed against the risk of inducing seizures.72
Although a majority of TNP patients receiving MCS achieve some measure of pain relief, it not clear why many suitable candidates fail to respond. To aid in patient selection, several investigators have attempted to correlate a variety of factors with MCS responsiveness. Yamamoto et al. reported that pain reduction with infused pharmacologic agents predicted pain relief by MCS; success was correlated with sensitivity to thiamylal and resistance to morphine.73 Katayama and colleagues underscored that integrity of the corticospinal tract is critical to MCS success; 15% of patients in one series achieved adequate pain relief when moderate or dense motor weakness existed in the affected territory and only 9% benefited when no motor contractions could be elicited (vs. a 73% success rate when motor impairment was absent or mild74). Analgesic effect has long been postulated to depend upon accurate placement of the stimulating electrodes.3,11 Nuti et al. found that pain relief at the conclusion of the first month of stimulation was a predictor of long-term relief.62 Finally, pain relief by repetitive transcranial magnetic stimulation may predict response to MCS.75,76
There have been no large controlled, prospective trials evaluating MCS for facial pain (or for any other chronic pain syndrome). A recent literature review identified reports of only 44 nonredundant cases of MCS for TNP.69 This is compounded by the fact that existing case series are heterogeneous with respect to patient population, inclusion criteria, surgical technique, stimulation parameters, outcome assessment, and a host of other factors. Concerns have also been raised of a possible bias toward the reporting of only case series with positive outcomes69 (see discussion of Brown and Pilitsis4). MCS is well-suited for randomized crossover trials with sham stimulation, as the stimulation rarely generates any perceptible sensation. And yet, few investigators have invoked this paradigm. The neurosurgical community now confronts a scenario similar to that seen after the initial reports of DBS for pain: An emerging technique is gradually adopted into widespread use without a rigorous vetting process. While MCS represents a promising therapy for TNP patients who have exhausted traditional treatment strategies, it merits formal interrogation in prospective, controlled trials.
Preoperative Considerations
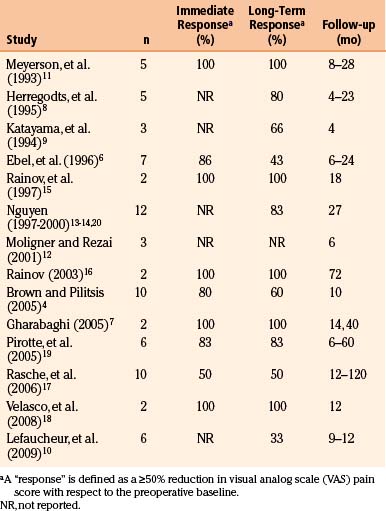
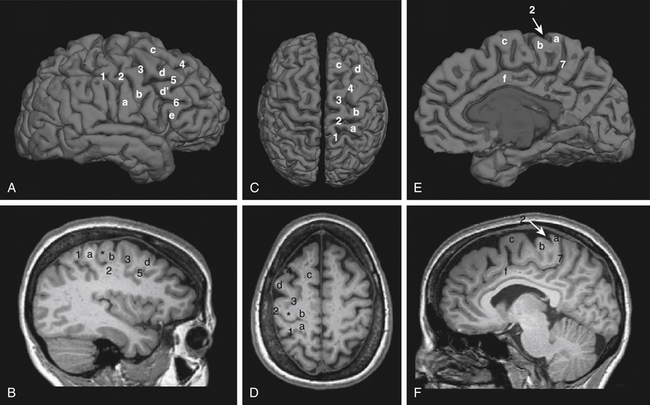
Preoperative planning begins with identification of the central sulcus and subsequently the face motor region. There are a variety of anatomic landmarks that can assist in identifying the central sulcus (Fig. 131-1).77–80 The central sulcus is immediately posterior to the precentral sulcus, which in turn marks the termination point of the horizontally oriented superior and inferior frontal sulci. The precentral sulcus is sometimes distinguished by an interruption by the middle frontal gyrus, but the central sulcus is usually continuous. The omega-shaped knob, best seen on axial images, marks the hand motor region, and its posterior boundary is the central sulcus (Fig. 131-D).80 This same region has a hook-shaped appearance in sagittal section (Fig. 131-1B). On sagittal images of the mesial wall of the hemisphere, the central sulcus can also be identified as the notch immediately anterior to the termination of the marginal branch of the cingulate sulcus at the vertex (Fig. 131-1E and F).78 In addition, the cortical thickness of the anterior (motor) bank of the central sulcus is approximately twice that of the posterior (sensory) bank on T2-weighted MRI images.77
Once the central sulcus and primary motor cortex have been identified, the face motor region can be determined. Early work has shown that the optimal stimulation target is in the precentral gyrus, approximately at the level of the inferior frontal sulcus.20 Due to individual variations in functional anatomy, however, other means of verifying the face representation within the motor strip can be valuable. Some studies have advocated for the use of transcranial magnetic stimulation (TMS) for this purpose. In TMS, a strong rapidly alternating magnetic field is focused on a cortical region, inducing a local current loop. Placement of the stimulator over the face motor cortex produces contractions of the contralateral face musculature. A few studies have demonstrated some analgesic benefit to patients with TNP from repetitive TMS (rTMS),81,82 and response to rTMS may predict a corresponding response to MCS.71
Individual localization can also be performed with functional MRI (fMRI). Blood oxygenation level–dependent (BOLD) fMRI, the most common form of the technique, relies on changes in local tissue oxygenation due to the hemodynamic response to increased neuronal activity. Voluntary tongue movement is a robust activator of the face motor region on fMRI. The fMRI can be co-registered with the patient’s structural MRI (Fig. 131-2) and imported into the intraoperative navigation system. We feel that using fMRI to help identify the face motor region is a helpful adjunct in preoperative planning and intraoperative navigation during MCS surgery, and others have also documented its utility.19
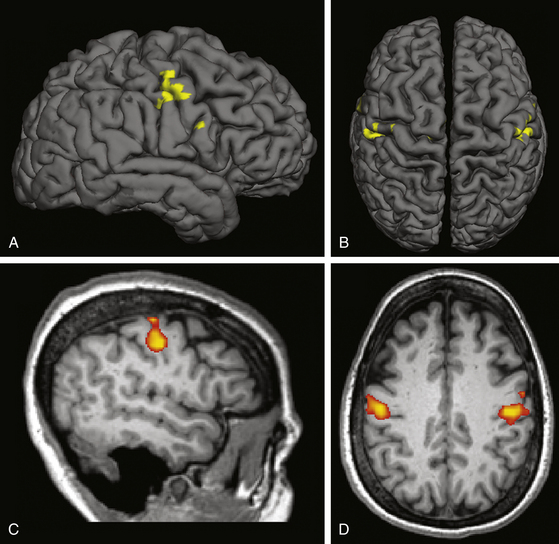
FIGURE 131-2 Functional MRI for face motor localization. This figure depicts an fMRI activation produced by face movement (volitional smiling) of the same patient as in Fig. 131-1. As is typical, the activation is centered within the depths of the central sulci bilaterally. A and C, Lateral surface and sagittal slice, respectively, of the right hemisphere. B and D, Vertex view and axial slice (in radiologic convention), respectively. The fMRI response can be superimposed on a surface registration of the patient’s brain, permitting individual localization of the target.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree