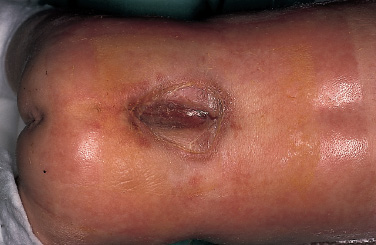
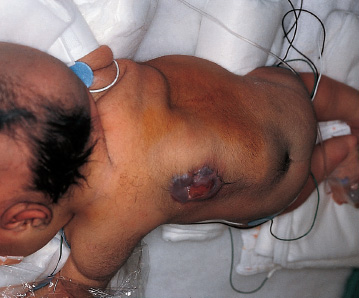
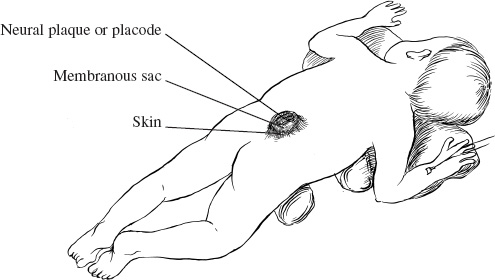
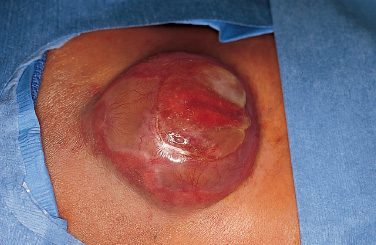
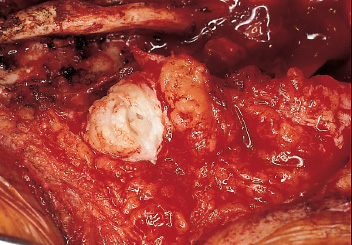
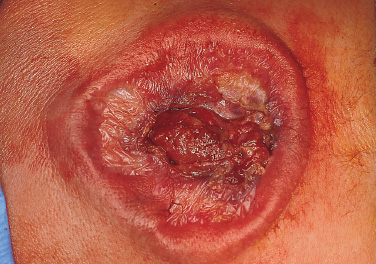
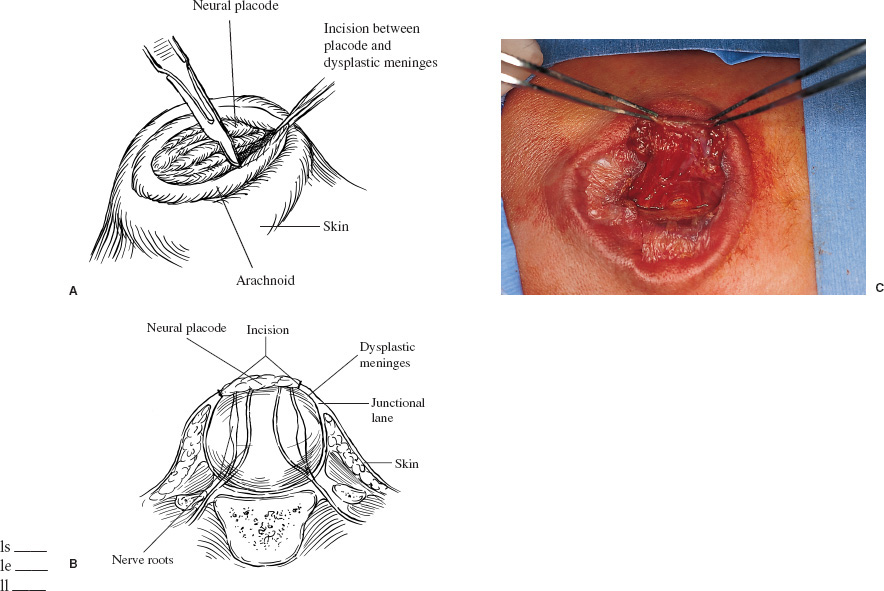
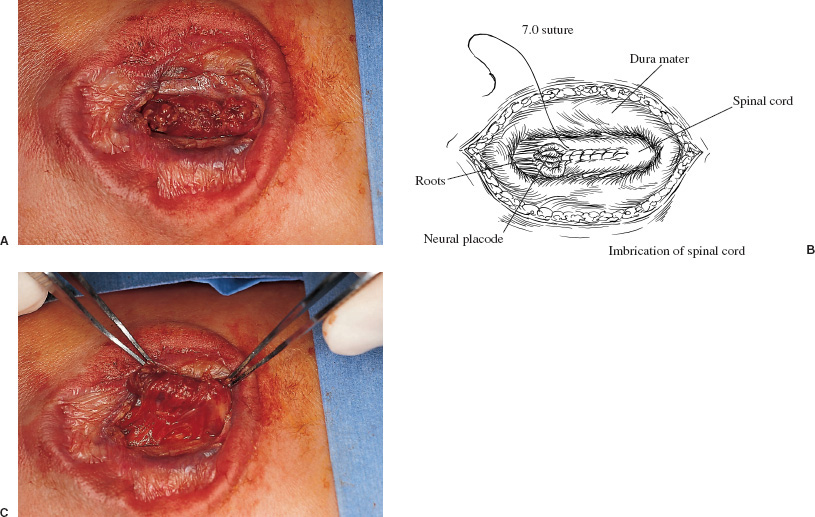
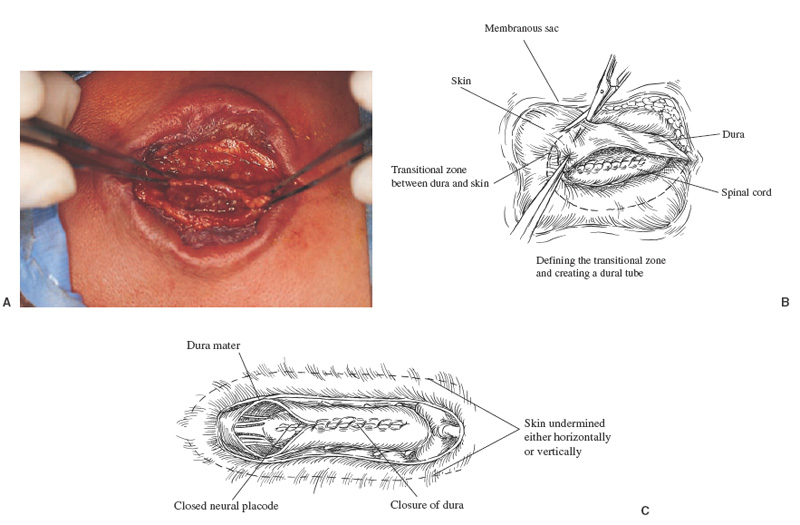
Patients with myelomeningoceles are often referred to as having spina bifida; however, the term spina bifida refers to a midline defect in the mesenchyma-derived tissues and does not include the neural tube (NT), which is the significant element. It is better to refer to these congenital lesions as neural tube defects (NTDs), which, from a practical, clinical standpoint, can be subdivided into two groups: open and closed. With an open NTD, visible neural tissue is present and cerebrospinal fluid (CSF) drains either continuously or intermittently from the lesion. The entire central nervous system (CNS) is affected with anomalies that include the Chiari II hindbrain malformation, polymicrogyria, and other lamination defects of the cerebral cortex. Hydrocephalus is commonly present, and more than 90% of these newborns require CSF diversion for control of its progression. The widespread CNS anomalies may relate to the early and continuous loss of CSF from the open NTD. With a closed NTD, no neural tissue is exposed, and CSF does not drain from the lesion. With few exceptions, such as some forms of posterior cervical meningoceles, the deformity of the CNS associated with a closed NTD is limited exclusively to the lower spinal cord. Open NTDs can be subdivided into three types. The most common is the myelomeningocele, which is cystic in appearance with a dorsally displaced neural plaque or placode setting atop a collection of CSF. The second type, much less common, is that of myeloschisis (Fig. 7–1), wherein the open NT is “plastered” against the anterior wall of the spinal canal; there being no cystic structure. The third, and quite rare, is the hemi-myelomeningocele (Fig. 7–2) wherein the spinal cord is split with one portion being an open NTD and the other closed. A cranial ultrasound, if convenient, can be done pre-operatively to gauge the ventricular size at that time. The ventricles are usually only mildly to moderately dilated, and rarely is the hydrocephalus marked as the loss of CSF at the site of the open NTD prevents the hydrocephalus from becoming progressive unless there is complete aqueductal stenosis. Advances in anesthetic management have made operating on a newborn a routine matter, with the incidence of complications becoming ever lower. The two areas that tend to count for most problems are heat loss and blood loss. There are several ways to keep the temperature of the newborn at the appropriate level. An important factor is to monitor the infant’s temperature and to make adjustments before the temperature drifts out of normal range. Because blood loss with most open NTD repairs can be kept to a small amount with judicious attention to hemostasis, usually only one peripheral intravenous line is needed. When a more extensive procedure is planned, such as a kyphectomy, a second intravenous and even an arterial line should be considered. When the blood loss reaches approximately 10% of the circulating blood volume, a decision needs to be made about possible transfusion, depending on how much more blood loss is expected before completion of the repair. The infant is paralyzed for induction but not thereafter, which allows one the ability to stimulate suspected functional nerve roots, if needed. FIGURE 7–1. A newborn with myeloschisis. Note that there is no cystic component to this open NTD. The open neural tube is displaced and lies anteriorly on the spinal canal. CSF drains from the central canal at its rostral end. Other than having less tissue to close, this defect has the same extensive CNS malformation as a myelomeningocele. FIGURE 7–2. A newborn with a hemimyelomeningocele. This open NTD is thoracic in location and to the left of midline. The spinal cord is split with one portion being open and the other closed. The left lower extremity has minimal motor sensory function, while the right was normal. The hemicord on the right is fully ensheathed with dura mater while the left hemicord had an exposed neural placode. The malformation of the CNS was the same as if this infant had a full myelomeningocele. If only the open NTD is to be addressed, the patient is placed prone, arms above the head, and towel rolls are placed under the upper chest and pelvis (Fig. 7–3). The lesion is cleaned with only normal saline or Ringer’s lactate, taking care to avoid agents that could damage exposed neural tissue (Fig. 7–4). An iodine-impregnated adhesive drape with a large hole in the center is used to secure the towels and is placed on the intact skin at some distance from the defect. We have not routinely inserted a CSF diverting shunt in an infant at the time of repair of the open NTD unless the hydrocephalus is marked. If both are to be done together while avoiding the need to reprepare and drape the infant, it is necessary to rotate the torso to have access to the peritoneal cavity as well as the back and head. The peritoneal tubing is directed medial to the scapula and enters the abdominal cavity from the flank. Visualization is enhanced with either higher-power loupe magnification or with an operating microscope. The only additional adjunct is the availability of a nerve stimulator. The goal of operative repair is to preserve the neural function and prevent infection, primarily by obtaining a good skin closure over the defect. The manner in which the open NTD is closed can prevent long-term secondary complications, such as an enlarging dermal inclusion cyst (Fig. 7–5), and may diminish the later effects of tethering. These lesions come in a spectrum as to size, shape, and complexity; no two are alike. A degree of flexibility is needed in the technique to obtain the best closure. The overwhelming majority of these lesions can be simply closed without the need for myofascial flaps, rotational full-thickness skin flaps, partial-thickness skin grafts, or flank-relaxing incisions. At the beginning of the procedure, no tissue is excised that might be used for closure at the end. The dysplastic epithelial tissue adjacent to the neural plaque provides a better source of skin than a partial-thickness skin graft taken from elsewhere. Newborn tissue has a remarkable healing capability if the blood supply is adequate and infection does not intervene. At the beginning of the procedure, CSF is often obtained and sent for a cell count with differential, gram stain and culture to be used as a baseline for future comparison, if needed (Fig. 7–6). The neural plaque is incised circumferentially, care being taken to exclude any epidermal or dermal elements that could subsequently develop into an inclusion cyst (Fig. 7–7). In many cases, it is possible to close the open NTD by bringing the lateral edges of the plaque to the midline and suturing the pial surface of the placode together with a fine, running, absorbable suture (Fig. 7–8). The configuration of some neural plaques make it difficult to obtain “a meaningful” closure of the NT. Any adhesions to the overlying meninges at the rostral end of the defect are divided. The dysplastic dura mater is circumferentially incised, taking care to have more than enough tissue to cover fully the repaired NTD and attached nerve roots with excess dura mater to spare because this might decrease the degree of adherence and thus later symptomatic tethering (Fig. 7–9). The dura mater is separated from the deep fascial layer beneath, brought together in the midline, and closed with a fine running, absorbable suture (Figs. 7–10 and 11). FIGURE 7–3. Salient features of the myelomeningocele. Patient is positioned for surgery with a small transverse roll under hips and another roll under chest. No attempt is made to mobilize the deep fascial layer, with or without muscle attachments, because to do so increases operative manipulation, tissue disruption, and blood loss without improving the repair or decreasing the incidence of skin dehiscence, wound infection, and CSF leakage. Occasionally, the gap in the deep fascial layer is small, in which case it can be approximated, assuming it does not compromise the neural structures in the spinal canal. If there are bony protuberances from the malformed posterior vertebral arch elements, these are separated from the surrounding soft tissues and removed to make a smooth surface, thereby decreasing the chance of possible compromise to the overlying skin. The skin is separated and undermined to a variable degree, depending on the size of the defect, at the abnormal junction between the cutaneous layer and the underlying deep fascia (Fig. 7–12). Skin closure is preferably done in a transverse rather than a vertical direction because to do so usually produces a better cosmetic result and keeps the incision further separated from the anus. However, whichever direction provides the least tension is generally chosen (Fig. 7–13). The superficial fascia is approximated to whatever degree possible, often with a gap between the two sides in the midportion of the defect, where it is widest (Fig. 7–14). FIGURE 7–4. Typical lumbosacral myelomeningocele which was prepped with normal saline prior to beginning the closure. FIGURE 7–5. A dermal inclusion cyst at the site of a previously repaired myelomeningocele. FIGURE 7–6. CSF has been drained from the sac and a sample sent for cell count with differential, gram stain and culture. FIGURE 7–7. A: Initial incision with #15 blade scalpel. Incision is made between the neural placode and membranous sac. B: The neural plaque is circumferentially freed from the surrounding dysplastic tissue with care being taken not to include any epithelial or dermal elements. Note the nerve roots attached to the neural placode. C: Placode separated from the membranous sac. CSF has been drained and the placode collapses toward the spinal cord. Redundant skin is removed only when the superficial fascia of the skin is approximated to the maximum degree possible, with one or two sutures at the point where the gap is the widest (Fig. 7–15). The amount excised from either side of the defect may be different, depending on the quality of the tissue. If necessary, all the dysplastic skin, including the thin membranous epithelium and arachnoid located just lateral to the plaque, is retained. This tissue readily heals and creates an intact epithelial barrier. At either end of the incision, the excess skin or “dog ears” are excised. At this point, the skin edges on either side of the wound should lie opposite one another with the minimal tension possible. Additional interrupted absorbable sutures are placed to approximate the subcutaneous tissue further. The cutaneous layer then is closed using a fine running, absorbable suture or Steri-Strip. FIGURE 7–8. A, B: The lateral edges of the neural placode are brought to the midline and closed with a fine absorbable running suture. This reconstitutes the neural tube and hopefully decreases the area for scarring and subsequent tethering. C: The re-constituted neural tube is shown with the nerve roots attached. Repair of a myeloschisis is similar to that of a myelomeningocele, the only difference being less dysplastic dura mater, superficial fascia, and skin available for closing the defect. In this situation, every available bit of tissue counts. It is also necessary to undermine the skin surrounding the lesion more extensively. A hemimyelomeningocele makes its presence known. It is located on one side of the midline, usually in the thoracic region, and is associated with a marked motor or sensory discrepancy deficit between the lower extremities (Fig. 7–2). This form of an open NTD is always associated with a split-cord malformation wherein the two cord segments are in separate dural sheaths with a bony spur between. In addition to repair of the open hemicord, the bony spur should be removed and the cord untethered at the time of the repair. In this situation, it would be helpful to perform magnetic resonance imaging (MRI). Although infrequent, other NTDs can be seen in conjunction with a myelomeningocele. The most common are a split-cord malformation with the two hemi-cords within the same dural sheath and a neurentenic cyst. Occasionally, the newborn might also have a marked gibbus deformity, which is usually seen in the context of a lumbothoracic or thoracic myelomeningocele, often with a complete loss of neurologic function below the level of the defect. In this situation, it is best to resect one or more vertebral bodies to reduce or eliminate the gibbus. Because this additional operative manipulation results in a more significant blood loss, transfusion should be anticipated. If the patient is to have a shunt placed at the same time as the open NTD is repaired, it is recommended that the shunt be placed first because theoretically there should be less chance for contamination of the shunt hardware and resultant infection. Once the shunt is in place, the patient can be partially repositioned to gain better access to the myelomeningocele without having to reprepare and drape the field.
MYELOMENINGOCELES
Intraoperative Techniques
Anesthesia and Positioning
Surgical Approach
Additional Operative Procedures
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


