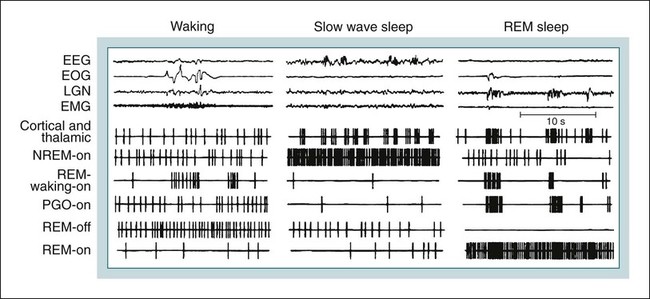
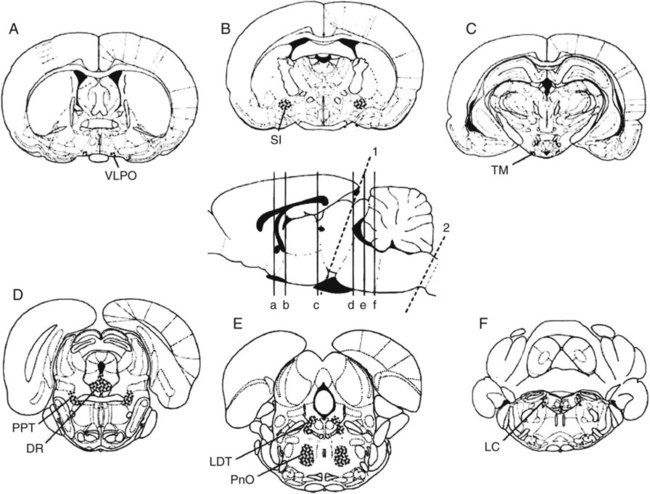
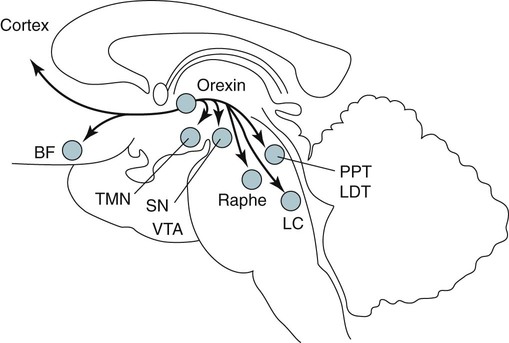
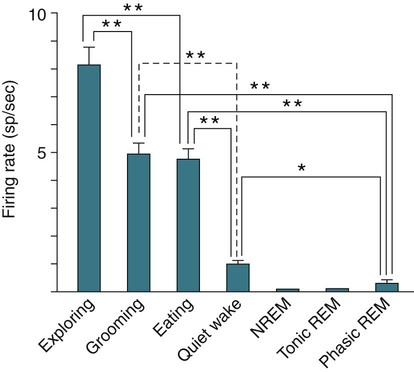
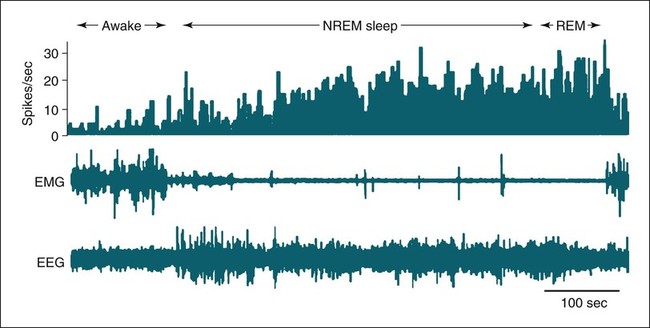
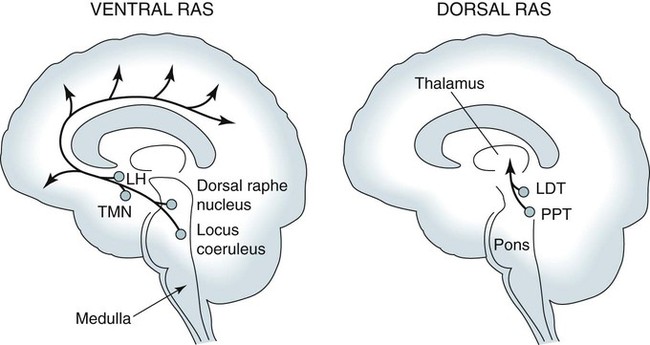
• Serotonergic neurons in the DRN are active during wake, less active during NREM, and even less active during REM sleep. • Noradrenergic neurons in the LC are active during wake, less active during NREM sleep, and inactive during REM sleep. • Histaminergic neurons in the TMN are active during wake and episodes of cataplexy. • Cholinergic neurons in REM-on areas are active during REM sleep. • Cholinergic neurons in wake/REM-on areas are active during wake and REM sleep. • Neurons in the VLPO are active during sleep and inactive during wake. They contain GABA and galanin. • Hypocretin neurons in the lateral and posterior hypothalamus project to many areas of the brain important for the control of wake and sleep. They stabilize transitions between wake and sleep. • Hypocretin neurons are absent (or very decreased) in patients with narcolepsy with cataplexy. These patients also have low or undetectable cerebrospinal fluid hypocretin-1. • The hypotonia of REM sleep is due to a combination of inhibition and dysfacilitation. • The brainstem regions involved in hypotonia include the subcoeruleus area (sublateral dorsal area), areas of the medulla and interneurons inhibiting the spinal cord motor neurons (glycine and GABA). In 1930, von Economo published findings of an autopsy study of the brains of patients dying from encephalitis lethargica.1,2 Von Economo found that patients with damage to the posterior hypothalamus and rostral midbrain often had excessive sleepiness, whereas those with injury to the anterior hypothalamus had unrelenting insomnia. Based on these observations, he hypothesized that the anterior hypothalamus contained neurons that promoted sleep, whereas neurons near the hypothalamus-midbrain junction helped promote wakefulness. Decades later, the importance of those areas of the brain for sleep and wake, respectively, has been increasingly understood. A number of major brain areas involved in control of wake and sleep, their abbreviations, and major associated neurotransmitters (neuromodulators) are listed in Table 7–1.3–6 TABLE 7–1 Brain Areas important for Sleep The term neurotransmitter is currently applied to situations in which one presynaptic neuron directly influences another postsynaptic neuron. In neuromodulation, a given neurotransmitter regulates the activity of diverse populations of neurons in the central nervous system. Examples of neurotransmitters that are also neuromodulators include acetylcholine (ACh), serotonin (5HT), dopamine (DA), and histamine (HA). Neurons are often characterized with respect to sleep by when they are most active.3 Some neurons are active during wake, during rapid eye movement (REM) only (REM-on), during REM and wake (wake/REM-on), during non–rapid eye movement (NREM) only (NREM-on), or during NREM and REM sleep (Fig. 7–1). Figure 7–2 shows sections through important brainstem areas involved in the regulation of wake and sleep and identifies important brain regions. Neurons in the lateral and posterior hypothalamus are the sole source of the awake-promoting neuropeptides hypocretin 1 (Hcrt1) and hypocretin 2 (Hcrt2), also known as Orexin A and Orexin B, respectively.7,8 Hcrt1 can attach to both Hcrt1 and 2 receptors, whereas Hcrt2 attaches only to Hcrt2 receptors. Patients with narcolepsy with cataplexy have loss of 90% or more of Hcrt-producing neurons and have low to undetectable cerebrospinal fluid (CSF) levels of Hcrt1.9,10 One study of a few patients with narcolepsy without cataplexy found partial loss of Hcrt neurons.11 Canine narcolepsy is due to a mutation in the gene for the Hcrt2 receptor. Hcrt neurons send abundant excitatory projections to the dorsal raphe (Hrct1 and Hcrt2 receptors) nucleus, the locus coeruleus (Hcrt1 receptors), and the tuberomammillary nucleus (Hcrt2 receptors) (Fig. 7–3). These areas in turn send inhibitory projections to Hcrt neurons. Hcrt neurons have a strong excitatory effect on the cholinergic neurons of the basal forebrain that contribute to cortical arousal but have no effect on GABAergic sleep-promoting neurons within the ventrolateral preoptic (VLPO) area. As is discussed later, Hcrt appears to stabilize transitions between wake and sleep. Hcrt neurons are relatively inactive in quiet waking but are transiently activated during sensory stimulation. Mileykovskiy and coworkers12 found that Hcrt cells are silent in slow wave sleep and tonic periods of REM sleep, with occasional burst discharge in phasic REM (Fig. 7–4). Hcrt cells discharge in active waking and have moderate and approximately equal levels of activity during grooming and eating and maximal activity during exploratory behavior. The authors of this study concluded that Hcrt cells are activated during emotional and sensorimotor conditions similar to those that trigger cataplexy in narcoleptic animals. The VLPO is an area in the hypothalamus containing neurons active during sleep. Most sleep-active neurons in the VLPO are believed to be active during both NREM and REM sleep (Fig. 7–5).13 Many of the VLPO neurons are activated by sleep-inducing factors including adenosine and prostaglandin D2. These neurons are sensitive to warmth, and heating this area of the brain increases their activity and decreases wake. A compact group of VLPO neurons (VLPO cluster) projects to the tuberomammillary nucleus (TMN) and inhibits the neuronal activity of that area. A second group of VLPO neurons is located dorsal and medial to the VLPO cluster neurons and the group is called the extended VLPO (eVLPO) by some authors.14 The eVLPO neurons make up the majority of the projections to the dorsal raphe nucleus (DRN) and locus coeruleus (LC) as well as to the interneurons of the lateral dorsal tegmental/pedunculopontine tegmental (LDT/PPT) region.14 One study showed a subset of neurons in the eVLPO were more active during REM than NREM.14 However, most VLPO neurons appear to be active during both NREM and REM. The neurons in the VLPO contain the neurotransmitters/neuromodulators gamma-aminobutyric acid (GABA) and galanin. The VLPO neuronal projections to the DRN, LC, and TMN are inhibitory (Fig. 7–6). The neurons in the VLPO receive inhibitory projections from the DRN, LC and TMN. Destruction of the VLPO impairs sleep. A group of sleep-active neurons in the median preoptic nucleus (MnPO) was described after the identification of the VLPO.13 Similar to VLPO cells, a subset of MnPO neurons expresses c-Fos and discharges more rapidly during NREM and REM sleep. Interestingly, unlike VLPO neurons, the MnPO cells also fire faster during prolonged wakefulness (which increases sleep pressure). There is no evidence that lesions of the MnPO area impair sleep. Histaminergic neurons are confined to the posterior hypothalamus in the area called the tuberomammillary nucleus. TMN neurons project to the cerebral cortex, amygdala, substantia nigra (SN), DRN, LC, and nucleus of the solitary tract. HA acting at H1 receptors is associated with wakefulness, and antihistamines (H1 receptor blockers) cause drowsiness or sleep. Conversely, H3 receptor agonists cause sleepiness possibly by stimulating autoregulatory receptors that decrease HA release. The TMN receives stimulatory input from the lateral hypothalamus (Hcrt). The TMN firing rate is high during wake, lower during NREM, and absent during REM.3 In contrast to REM sleep, during attacks of cataplexy, TMN neurons have a high firing rate associated with preservation of consciousness.15 Low CSF HA has been found in patients with narcolepsy with and without low Hcrt.16,17 The low HA may be a marker rather than a cause of sleepiness because lesions of the TMN have minimal effect on wakefulness. However, this may simply mean that HA is not essential for wakefulness in general. HA may be important at the onset of wakefulness. Neurons producing dopamine (DA) are abundant in the SN and ventral tegmental area (VTA). Previously, studies suggested that DA neurons do not change their firing rates substantially across sleep stages.3 However, extracellular DA levels are high in several brain regions during wakefulness. Whereas the average rate of firing of the VTA neurons does not change across states, they have burst firing during wake that releases more DA. Recent work has identified DA cells in the ventral periaqueductal gray (vPAG) that are wake active.18 The vPAG contains DA neurons that are active (express Fos) during wakefulness but not sleep. DA neurons in the vPAG project to and receive input from cholinergic neurons in the LDT area, Orexin neurons, VLPO, and prefrontal cortex. Loss of vPAG DA neurons promotes sleep. The reticular formation is a loose collection of neurons extending from the caudal medulla to the core of the midbrain. Sections above the mid pons produce coma or hypersomnolence. Wakefulness depends on the activity of the ascending reticular activating system (ARAS). This system projects to higher brain centers. One pathway ascends dorsally to the thalamus, and the second ascends ventrally through the lateral hypothalamus and forebrain (Fig. 7–7).
Neurobiology of Sleep
AREA
ABBREVIATION
NEUROTRANSMITTER
Lateral dorsal tegmentum
LDT
Acetylcholine (ACh)
Pedunculopontine tegmentum
PPT
Acetylcholine (ACh)
Locus coeruleus
LC
Norepinephrine (NE)
Dorsal raphe nucleus
DRN
Serotonin (5HT)
Tuberomammillary nucleus
TMN
Histamine (HA)
Ventrolateral preoptic area
VLPO
Gamma-aminobutyric acid (GABA) Galanin
Lateral posterior hypothalamus
LPH
Hypocretin 1 and 2 (Orexin A, B)
Major Brain Areas Important for Sleep and Wake
Hypothalamic Areas
Lateral Hypothalamus
Ventrolateral Preoptic Nucleus
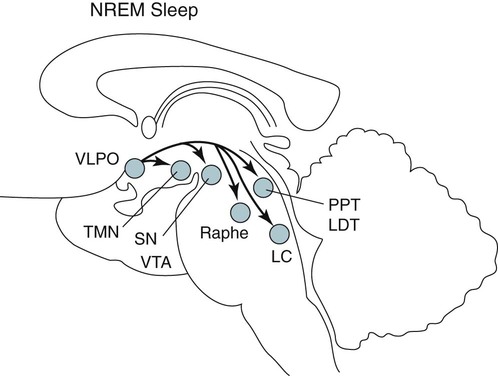
Tuberomammillary Nucleus
Brainstem Regions
Dopamine Regions
Reticular Formation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurobiology of Sleep





