Neurocognitive Processes and the EEG/MEG
Fernando H. Lopes Da Silva
INTRODUCTION
The interest in using electroencephalography (EEG) signals to understand cognitive processes has been a permanent question since the days when Berger recorded EEG signals for the first time. According to Niedermeyer (1), Berger’s driving force “was the quest for the nature of the all-powerful force of mental energy (Psychische Energie).” Subsequently, many researchers pursued this question and tried to use EEG signals to understand the material basis of cognitive processes. Basically, the strategy followed by most researchers in this field has been essentially correlative. Many EEG studies in the field of neurocognition concern averaged evoked or event-related potentials or magnetic fields (ERPs or ERFs); in parallel investigations of changes of spontaneous ongoing EEG/MEG signals induced by particular cognitive events are also actively pursued mainly in the last decades. Since the former are the subject of a number of specialized chapters in this book, we focus here on the latter.
In most studies, a specific task is designed, usually according to a more or less standard psychological paradigm, while simultaneously EEG or MEG signals are recorded. Thereafter researchers extract some properties of these signals and use statistical approaches to find significant associations between cognitive and EEG features. These kinds of investigations are based on a number of assumptions: (1) sensory processing, motor programming, and specific cognitive functions (perception, attention, memory, decision making) depend on patterns of neuronal spike firing; (2) neuronal spike firing is reflected, at least partially, in local field potentials (LFPs) according to biophysical rules that account for field electrical potentials and magnetic fields that result from soma-dendritic membrane processes, voltage-dependent membrane oscillations, afterpotentials, and synaptic activity; and (3) LFPs are the basic building blocks of EEG/MEG signals (and also of functional magnetic resonance [fMRI] signals as we briefly discuss later).
It should be emphasized, however, that not all neuronal activities can be detected at the level of EEG signals, both recorded at the scalp or even intracranially. In addition the detection of clear relationships between neuronal activities and cognitive processes is not simple since relevant neuronal properties underlying cognitive processes may elude the methods currently used to detect them, both in human and in animals. For example, it is difficult to sample brain space in a satisfactory way in order to detect a complete set of neuronal activities underlying a given cognitive process, in spite of outstanding advances in electrophysiologic and magnetoencephalographic technologies, namely high-density whole-head, or intracranial multiple macro- and microelectrode recordings (e.g., tetrodes). This limitation in spatial sampling can be partially compensated by simultaneous recordings of EEG and hemodynamic signals by way of functional MRI (blood oxygen level dependent or BOLD).
Basic neurophysiology using microelectrode recordings of action potentials (APs) especially in behaving animals has been a most valuable source of information regarding the coding of sensory information and the physiology of motor systems with a degree of detail that EEG/MEG recordings cannot reach. Nonetheless brain functions involve always the activity of populations or assemblies of neurons that form dynamical networks with varying degrees of coupling. These dynamical assemblies of neurons constitute the basic units of brain functioning responsible for cognitive processes. It is at this level that EEG/MEG signals are particularly relevant since they can reflect the activity of these neuronal assemblies and their dynamic relationships. fMRI can yield additional and very relevant information about the degree of activity of different structures throughout the whole brain; interestingly, it was shown that this BOLD signal corresponds closer to the LFPs, which form the local sources of EEG signals, than to the density of trains of APs (2,3). It may be emphasized that there are appreciable differences between EEG/MEG signals and BOLD signals regarding time and spatial scales. The time resolution of the former is in the order of milliseconds while that of the latter is in the order of seconds. The spatial resolution is also different; the unitary module responsible for the weakest measurable cortical MEG signal is in the order of 10 nAm, which corresponds to about 50,000 neurons, taking into consideration that the current dipole of a cortical pyramidal cell is in the order of 0.2 pAm/cell (4); a population of neurons of this size would correspond to a cortical patch with about 0.63 mm2 area; in contrast a typical unfiltered fMRI voxel (55 mL in size) contains about 5.5 million neurons (3). fMRI has, of course, the most important advantage of providing information about the whole brain in a direct way, without the limitation of having to estimate approximate solutions of the inverse problem in contrast to EEG/MEG. Furthermore, the latter gives information predominantly about the cortex; in very simplified words, EEG reflects mainly the activity of the crown of gyri and MEG of the fissural cortex (see Chapters 5 and 42). Nonetheless, EEG/MEG can detect phasing or timing of coherent neural activity throughout the cortex at the time scale at which cognitive processes evolve, which is an important asset.
The difference in strong points between EEG/MEG and fMRI makes that a combination of EEG and fMRI has the potential of contributing to unravel both the dynamics of neurocognitive processes and the brain structures involved in their realization if applied in an appropriate way.
THE FORMATION OF NEURONAL ASSEMBLIES AND THE GENERATION OF OSCILLATIONS
As stated above, a common assumption in the neurosciences is that neurons do not work in isolation, rather they form dynamical assemblies that work in near synchrony in order to realize determined functions. This assumption stems from the classic concept of Hebb (5) who postulated that neuronal assemblies are dynamical systems with plastic properties that subserve learning and memory. These assemblies may vary in dimension and in degree of synchrony. A multitude of neuronal assemblies are usually simultaneously active in the brain, such that we may state that the brain constitutes a complex system where parallel processing, under normal conditions, is constantly going on. These neuronal assemblies may occupy different cortical areas that are not necessarily anatomically contiguous. It is therefore necessary for the brain to integrate the operations of multiple neuronal units and neuronal assemblies. In other words, the brain has to integrate distributed sets of neuronal activities spread over multiple cortical macrocolumns, to achieve coherent representation of events and to effectuate coordinated actions. This essential property of the brain has been analyzed by means of connectionist models based on parallel computing (6) and interpreted in terms of the theory of neuronal group selection (7). An interesting experimental contribution to understand how distributed sets of neuronal activities can generate coherent activity has been provided by the observations of Vaadia et al. (8) who recorded small populations of single units (6, 7, 8, 9 and 10) in the cortex of cats and monkeys during several behavioral conditions. A salient finding was that information processing in the cortex is represented by coactivation of sets of neurons rather than by independent modulation of the single-unit firing rate. Furthermore, single neurons may participate in more than one functional set, and each small cortical area may contain members of several functional sets. The strength of the coactivation among adjacent neurons can be modulated by sensory stimulation and by arousal. This coactivation between neurons is reflected in the properties of LFPs.
WHAT ARE THE MECHANISMS UNDERLYING COACTIVATION AND THUS ARE RESPONSIBLE FOR THE FORMATION OF DYNAMICAL NEURONAL ASSEMBLIES?
In general, the neurons that constitute those assemblies, main cells, and interneurons are interconnected by feedforward and feedback loops. The dynamical behavior of these assemblies depends mainly on local feedback and feedforward gains, rise, and decay of synaptic potentials, time delays, and kinetics of ionic conductances. These intrinsic neuronal properties are controlled by neuromodulatory parameters and biochemical variables. Not surprising the existence of these loops favors the occurrence of oscillations; furthermore, many neurons, given the appropriate conditions, have intrinsic oscillatory properties that facilitate, or reinforce, the tendency for neurons in these loops to oscillate collectively. Oscillatory behavior is not a prerequisite for the formation of neuronal assemblies, but it certainly facilitates their formation.
DO NEURONAL OSCILLATIONS HAVE FUNCTIONAL SIGNIFICANCE?
There are many instances of oscillations occurring in nervous systems from the simpler organisms to the more complex ones. A comprehensive review of oscillations in nervous systems, from the level of cultured neural networks and invertebrate ganglia, to the more complex level of the mammalian brain can be found in the book edited by Levine et al. (9), at the molecular, cellular, synaptic, and network levels. A characteristic property of these neuronal assemblies is that they display nonlinear dynamical behavior. Thus, we should be aware that neuronal oscillations constitute a biologic property that has been conserved in the course of evolution. Rhythm-generating circuits can be very flexible in responding to external stimuli and to modulating influences from various sources. An interesting system where the importance of oscillations and neural synchronization has been put clearly in evidence in processing sensory information (10) is the insect olfactory system where the antennal lobe generates oscillatory synchronization of its output as a framework for coincidence detection by its target structure, the mushroom body. These oscillations appear to play a role in a form of spike timing-dependent plasticity that promotes the propagation of odor information through the olfactory system (11). Furthermore, the basic design of neural circuits displaying synchronized oscillatory activities appears to be used throughout nervous systems including the human brain. In this context, it is interesting to mention that Marder (12) points out that the circuits controlling vertebrate respiration show considerable similarity in design to the pyloric network of the crustacean stomatogastric ganglion.
In the brain many kinds of generators of rhythmic activities exist (13). These rhythmic activities can become manifest in the form of oscillations of LFPs at various scales. Some of these are very local, but some are manifest as mass field potentials, ultimately contributing to the electrocorticogram (ECoG) and the EEG or MEG.
At this stage, we should put forward the general question whether neural oscillations are essential for brain functioning or not. In an interesting analysis of this question from a theoretical perspective, Friston (14) starts from the general concept that integration among neuronal populations uses transient dynamics that represent a temporal “code.” He distinguishes two possible kinds of transients in a set of neuronal assemblies—synchronous or asynchronous—and notes that synchronization may in turn be oscillatory, or not. This is the case of neuronal responses that encode a sensory stimulus by
discharging in synchrony as proposed by Singer (15) and Singer and Gray (16). Accordingly Friston distinguishes between oscillatory codes and nonoscillatory codes. This implies that synchronized oscillations are not the unique form of “coding” information in the brain; they are just one form, even though a very salient one. We should note that there has been a debate (17) about the prevalence of gamma oscillations and synchronization as carrying information about a percept; an insightful discussion of this debate is given by Gray (18) and we cannot go here into details, but below we consider some alternative forms of coding that are also relevant (19).
discharging in synchrony as proposed by Singer (15) and Singer and Gray (16). Accordingly Friston distinguishes between oscillatory codes and nonoscillatory codes. This implies that synchronized oscillations are not the unique form of “coding” information in the brain; they are just one form, even though a very salient one. We should note that there has been a debate (17) about the prevalence of gamma oscillations and synchronization as carrying information about a percept; an insightful discussion of this debate is given by Gray (18) and we cannot go here into details, but below we consider some alternative forms of coding that are also relevant (19).
In this chapter, we consider especially processes that belong to the category of “oscillatory coding” since we deal here with neural phenomena at the macroscopic level where rhythmic activities are dominant (EEG/MEG). This reference to Friston’s taxonomic classification of neural codes serves to underline that at the EEG/MEG level only a part of the realm of neural coding processes can be detected.
A direct relation between EEG oscillations and perception emerges from the experimental findings of Smith et al. (20) who tested the hypothesis that synchronized discharges of cortical cell assemblies with a prominent rhythm are the neural correlates of discrete cognitive and perceptual conscious states, as stated by VanRullen and Koch (21). They did this with an ingenious experiment. They let the subject look at a famous painting of Dali (The Slave Market with the Disappearing Bust of Voltaire of 1940) at the same time as the EEG was recorded. The painting is ambiguous, since one may perceive either the bust of the philosopher Voltaire or the pictures of two female figures dressed as nuns; they used this ambiguity to check at what time the subjects experienced either one or the other of the two distinct percepts and determined the corresponding most sensitive EEG features, that is, they related the dynamics of sensory information processing with the dynamics of brain states defined by EEG features, centered on three frequency bands: theta, 4 to 8 Hz; alpha, 8 to 12 Hz; and beta, 12 to 25 Hz, recorded from centroparietal electrode Pz. They found that the perceptual moment at which the subject recognized one of the visual features corresponded to a maximum of theta oscillations, whereas for the other visual feature, the perceptual moment corresponded to beta oscillations. We concur with the authors’ conclusion that “the evidence reported here supports the idea that oscillatory brain activity underlies the processing of visual information subtending the perceptual moments of conscious visual experience.” These findings reinforce the notion that oscillatory coding plays a role in perception, which is also supported by other experimental observations, among others those of Rodriguez et al. (22), Trujillo et al. (23), and Doesburg et al. (24), described below.
OSCILLATIONS: FROM NEURONAL FIRING THROUGH LFPS TO EEG/MEG SIGNALS
Taking into consideration that LFPs are the building blocks of EEG (MEG) signals, it is logical to examine, first, experimental evidence that relates LFPs to spike firing and behavior. Indeed several studies showed evidence that LFPs can carry information about a given stimulus or a specific behavior. In a well-controlled investigation of the effect of a visual scene (a movie) on the visual cortex of monkeys, Montemurro et al. (25) quantified both spike counts and the low-frequency LFP, which was phase modulated by the presentation of the movie. This visual stimulus elicited higher firing rates, whereas the LFP phase became highly reliable across trials. These authors found that the LFP phase related to neural firing conveyed additional information beyond that conveyed by spike counts and concluded that “phase coding may allow primary cortical neurons to represent several effective stimuli in an easily decodable format.” The results of this study demonstrate that LFPs can promote the synchronization of neuronal firing that becomes apparent in the phase relation between LFP and unit firing. Also in relation to motor programming the same kind of relation between LFPs and spike firing has been shown to exist. As described later in the subsection on beta rhythms, Murthy and Fetz (26) found that in the motor cortex the cortical neurons can become transiently synchronized specifically during LFP oscillations, even if their spikes are uncorrelated during nonoscillatory periods, which indicates that the oscillatory behavior plays a role in promoting the synchronization of the neuronal units. Also in the visual system Fries et al. (27) found increases of the correlation between spikes of single neurons and LFPs in a task involving visual attention. In a comprehensive review of the functional significance of LFPs, particularly in the gamma frequency range, Fries et al. (28) suggest that the adjustment of spike timing by the gamma cycle is not an epiphenomenon but a fundamental mechanism in cortical information processing. A number of similar correlates that reinforce the statements presented here can be found among others in the review of Mazzoni et al. (29).
Thus we may conclude, based on these experimental findings, that LFPs are not only related to spike firing but they are able, next to spikes, to carry additional information about specific stimulus or neuronal programs. We may state further that LFPs are appropriate variables in order to relate neuronal activity to sensory and motor programming, that is, to cognitive processes.
EEG/MEG oscillations occur at different frequencies from the infraslow, say 0.5 Hz, to the very fast, reaching values of several hundreds of hertz. In general, oscillations at the lower end of the frequency spectrum tend to engage larger spatial domains, whereas those at higher frequencies are localized to restricted cortical areas. In general terms, and at the cost of oversimplifying, we may state that the former, the delta waves, are particularly suited to set a functional bias throughout a large population of neurons, as occurs in “up-states” of slow wave in sleep. Oscillations at intermediary frequencies, such as in the theta and alpha ranges, are optimal to modulate the transfer of information across specific populations, such as those of the hippocampal formation and associated cortical areas in the case of theta and of thalamocortical (TC) systems as in the case of alpha. Oscillations at the higher frequencies, in the beta and gamma range, are specially adequate to engage relatively discrete populations in achieving transfer of packets of specific information among neuronal assemblies.
Notwithstanding the inevitable loss of information that takes place when one goes from the level of local neuronal
assemblies to the level of the EEG/MEG, successful attempts have been made to find correlations between EEG signals, occurring during a particular behavior, in order to infer which brain systems are responsible for such a behavior and how they are functionally related, for instance, during the preparation and performance of accurate visuomotor behaviors (30,31) and cognitive tasks (32). We have to accept, however, that ongoing EEG/MEG activity may only yield limited insight in how information is processed in the brain.
assemblies to the level of the EEG/MEG, successful attempts have been made to find correlations between EEG signals, occurring during a particular behavior, in order to infer which brain systems are responsible for such a behavior and how they are functionally related, for instance, during the preparation and performance of accurate visuomotor behaviors (30,31) and cognitive tasks (32). We have to accept, however, that ongoing EEG/MEG activity may only yield limited insight in how information is processed in the brain.
Nonetheless we may state that ongoing EEG/MEG signals can be useful as bridges between activities at the neuronal level, about which it is very difficult to gather information in normal human subjects, and behavioral and cognitive phenomena.
CONTRIBUTION OF EEG/MEG ANALYSIS TO NEUROCOGNITIVE STUDIES: SKEPTICISM AND REALISM
We have to note that some scientists may be skeptical about the value of neuronal network oscillations and EEG/MEG rhythmic activities for neurocognitive studies. For instance in their insightful review of “Network Oscillations,” Sejnowski and Paulsen (33) state that notwithstanding “extensive work on the behavioral and physiologic correlates of brain rhythms, it is still unresolved whether they have any important function in the mammalian cerebral cortex.”
We acknowledge that there may be reasons for this skepticism, particularly in face of many simplistic claims of correlates between gross EEG features and cognitive processes. Nonetheless, the evidence that has been built up in the recent decades provides a realistic basis to propose that it is worthy to analyze neuronal activities underlying cognitive processes at three levels of complexity: microscopic (spike trains), mesoscopic (LFPs), and macroscopic (EEG/MEG) in order to advance in understanding how cognitive functions arise as emerging properties dynamical brain systems. We discuss here experimental evidence that justifies the statement that EEG/MEG features, notably certain rhythmic activities, are closely associated with cognitive processes and consequently can provide useful windows into cortical functional states underlying relevant cognitive processes.
THE USEFULNESS OF COMPUTATIONAL MODELING
In the context of this introduction, it is important to stress the need to use computational models by means of which the integration of structural, physiologic, and behavioral data may be realized at different levels of complexity, namely the microscopic, the mesoscopic, and the macroscopic levels. At the former, the fundamental processes of the function of neurons with their structural characteristics and processes of interneuronal communication can be quantitatively analyzed within the frame of the formation of small-scale neuronal assemblies; the activity of the latter can be analyzed as multiple spike trains. At the mesoscopic level, the properties of neuronal assemblies, forming functional cortical columns, that underlie cortical specific functions can be integrated; the latter can be reflected in LFPs. At the macroscopic level, insight can be obtained by analyzing the dynamics of such assemblies at the scale of the whole brain through the properties of the corresponding EEG/MEG signals, in relation to the features of emergent behavior (cognitive domain).
As stated in Chapter 4, the complexity of EEG phenomena calls for the use of mathematical models and computer simulations in order to understand the underlying processes of generation of these signals. In that chapter, a number of models encompassing these three levels of complexity are discussed in relation to processes responsible for the generation of different kinds of oscillatory phenomena as recorded in the EEG/MEG. In this context, it is also interesting to consider the models that have been proposed (34,35) in order to account for the whole spectrum of MEG/EEG signals based on oscillatory regime of nonlinear lumped models as originally developed by Lopes da Silva et al. (36,37), modified and refined by Jansen and Rit (38) and later by Wendling et al. (39) by simply changing population kinetics. These dynamic causal models (DCM,34) of neural assemblies are used to examine the influence of coupling strength and propagation delay on the rhythms generated by coupled cortical areas. Model studies showed that both coupling and propagation delays are critical determinants of the MEG/EEG spectrum and that it is possible to derive physiologically valid basis functions for statistical modeling of responses evoked by sensory stimuli. More recently, this model approach has been extended in an attempt to integrate EEG/MEG and fMRI phenomena using the same basic neuronal sources. Valdes-Sosa et al. (40) proposed a model-driven EEG/fMRI fusion approach with a focus on brain oscillations.
With respect to computational approaches of a wide range of brain functions (cells, circuits, brains, plasticity, and behavior) including sensorimotor integration, a comprehensive overview can be found in Churchland and Sejnowski (41).
THE SELECTION OF EEG/MEG FEATURES AND NEUROCOGNITIVE STUDIES
A basic question that has to be considered is which EEG/MEG features are relevant for this kind of studies. Traditionally, power spectra at different frequency bands are used. The rationale for the subdivision of the EEG in different frequency bands is described in several chapters of this book. In order to draw relevant inferences from such studies, it is necessary to be able to link the activities in these frequency bands to processes at the neuronal level. This is still only partially possible at this moment.
Here we have to make a note regarding the EEG variables that are mostly used in correlative neurocognitive studies. In general, these EEG phenomena are induced by the cognitive task. This implies that these activities are not time-locked to a stimulus and thus cannot be quantified by simple averaging. Quantification is commonly done by computing power spectral densities. The main EEG variables are the power in distinct frequency bands, the dominant spectral frequency, the
relationships between EEG signals recorded from different sites, particularly using cross-spectra, coherence, and phase functions, both in the linear and the nonlinear domains, and the estimation of equivalent brain sources. We should keep in mind that what one can record at the level of the scalp represents the activity of relatively large populations of neurons.
relationships between EEG signals recorded from different sites, particularly using cross-spectra, coherence, and phase functions, both in the linear and the nonlinear domains, and the estimation of equivalent brain sources. We should keep in mind that what one can record at the level of the scalp represents the activity of relatively large populations of neurons.
In many EEG studies, the spatial precision of the recordings is limited since the number of electrode sites used is not sufficient for adequate spatial sampling of some EEG features such as short bursts of gamma oscillations. Another factor that limits the interpretation of these data is the influence of volume conduction. In neurocognitive EEG studies in particular it is important to achieve an appropriate spatial sampling as well as adequate control of volume conductor effects. One example of how these effects may compromise the interpretation of EEG measurements are those studies of coherence and phase functions in which EEG signals are recorded against a common reference. In general, it is preferable to use several reference derivations and even better to use Laplacian derivations with appropriate spatial sampling (42).
The estimation of the localization of the sources of EEG phenomena induced by cognitive events is a difficult problem. These kinds of EEG oscillations have no strict phase relation with a stimulus and cannot be quantified by simple time averaging. Therefore, these oscillations have to be quantified in the frequency domain by spectral density. We should note that it is possible to make this estimation based on single trial data, if the signal-to-noise ratio is appropriate, for instance by using a time-varying dipole model to calculate, for selected sample points of the oscillations, the corresponding equivalent dipoles in a realistic model of the head; the source space is then estimated as constituted by those voxels where a significant number of equivalent dipoles are encountered, as done by Manshanden et al. (43) for alpha/mu rhythms and sleep spindles. To avoid the limitations imposed by the division of the EEG in classic frequency bands, a powerful alternative is the method of independent component analysis (ICA) coupled to time/frequency analysis, particularly at the level of single trials, as proposed by Makeig et al. (44,45). This method that is especially suited for studies of the association between EEG and cognitive processes is described in Chapter 54. Shortly it consists of a linear decomposition of EEG signals into a sum of components that contribute distinct information to the signal analyzed in an optimal way. The spatial distribution of the components can be visualized as scalp maps that can be used to estimate the corresponding brain sources and further characterization in the time-frequency domains can be carried out. The ICA method is particularly adequate as an analytical tool to investigate EEG/MEG signals on a trial-by-trial basis. One may hope that the application of ICA may be able to separate EEG/MEG signals into physiologically distinct sources, but whether or not this may be achieved is not immediately obvious and needs further analysis and interpretation. Furthermore, ICA can identify and separate the contributions of stereotyped extracerebral artifact signals including eye movements, line noise, and muscle activities, which is of great practical value in neurocognitive EEG studies. Makeig et al. (45) use the event-related spectral perturbation (ERSP) to measure event-locked changes in spectral power (Fig. 50.1) (45).
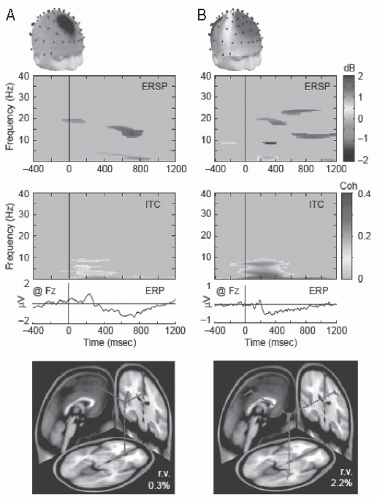 Figure 50.1 Maximally independent EEG components compatible with a single cortical source. Scalp maps, event-related spectral perturbation (ERSP) and inter-trial phase coherence (ITC) images, and event-related potentials (ERPs) for two typical independent EEG processes during visual letter encoding (green in images indicates P < 0.001). Below are the best-fitting equivalent current dipole (ECD) models (“r.v.” is the residual scalp map variance across the 69 scalp electrodes). A: Frontal midline theta component with radial ECD. Spectral power increases shown in the ERSP accompany weak phase locking on ITC and slow ERP effects (mV as at Fz). B: Mu-rhythm component with tangential ECD in right somatomotor cortex. Alpha- and beta-band (ERSP) power changes, accompanied by partial phase locking (ITC) contributing to the component ERP. (Adapted with permission from Makeig S, Debener S, Onton J, et al. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8(5):204-210.) (See color insert) |
A most important aspect of cortical neurophysiology within the framework of the relationships with cognitive processes is the fact that EEG/MEG rhythmic activities are distributed over the cortex not necessarily forming continuous spatial domains. This implies that it is necessary to extract information from these signals about associations between signals recorded from different sites. In this respect in Chapter 56 a number of appropriate techniques are described that are being currently applied. In general, two concepts of “association” and “time or phase relations” must be examined carefully. Quite commonly, the
term “phase synchronization” is used in this field. We prefer to talk about “phase relations” since this is a more general concept that permits the estimation of time relations between different cortical sites and thus to estimate flows of activities, sometimes called “flows of information.” In the past decades, many EEG neurocognitive studies have emphasized the importance of the estimation of phase synchrony as a relevant feature to reveal neurocognitive correlates (46).
term “phase synchronization” is used in this field. We prefer to talk about “phase relations” since this is a more general concept that permits the estimation of time relations between different cortical sites and thus to estimate flows of activities, sometimes called “flows of information.” In the past decades, many EEG neurocognitive studies have emphasized the importance of the estimation of phase synchrony as a relevant feature to reveal neurocognitive correlates (46).
In this context the concept of “brain workspace” may be useful. This term was coined by Baars (47) and used by Dehaene et al. (48) to account for the fact that the brain, besides the modular organization of specialized systems, consists also of distributed neural systems that form functional assemblies interconnected in a dynamical way. Such a dynamical distributed neural system is called a “workspace.” Further this concept implies that a functional link should exist between “brain workspace” and a cognitive event. This is the kernel of the workspace hypothesis as formulated by Dehaene and Naccache (49).
Another feature that is relevant in this context is the fact that different frequency components may not be independent. Below we will consider this feature with respect to the fact that some EEG signals in the alpha frequency range have higher harmonics due to the fact that the generators have nonlinear properties and that there may exist concatenation of different EEG components.
MAIN NEUROCOGNITIVE CORRELATES OF EEG FREQUENCY BANDS
Steriade et al. (50) reviewed the basic neurophysiology of EEG rhythms in relation to basic neuronal processes and behavior. According to the knowledge available at that time, the neurophysiologic processes responsible for some EEG specific frequency bands were put in evidence. Along the same line, a short review of the main neurophysiologic properties underlying different EEG frequency bands is presented here, as far as these are relevant to studies of EEG-cognitive relationships. As in the review of 1990 mentioned above in this chapter, we choose to present this analysis according to the main EEG/MEG frequency bands as is common practice in the literature. It is important, however, to note that different frequency bands are not independent variables and the same cognitive process may be associated with changes of EEG/MEG signals at different frequencies. Furthermore, there are interesting concatenations between frequency components that are relevant correlates of cognitive phenomena. This aspect is considered separately below.
Low-Frequency Activities, Excitability Cycles, Perception, and Memory Processes
In the past decades considerable advances were made regarding the neurophysiologic basis of EEG low-frequency activities, for example, between 0 and 4 Hz, the basic properties of which are discussed in Chapter 3. Definitions based exclusively on frequency bands may conceal underlying mechanisms. Thus, this EEG low-frequency band, that traditionally is called the delta frequency band, may comprise different electrophysiologic processes with distinct basic mechanisms. Studies in the last two decades have unveiled the electrophysiologic substrates of several distinct activities in the frequency range 0 to 4 Hz during sleep and anesthesia, but also in awake state. Basic ionic currents and intrinsic membrane phenomena underlying slow waves and oscillations are described in Chapter 3. It suffices to summarize here very briefly the main neurophysiologic features that characterize the EEG patterns of sleep.
The slow oscillations with a dominant frequency around 0.8 Hz are generated in the cortex. Since the work of Steriade (51) and collaborators we know that the slow waves consist of a succession of the so-called “down-states” and “up-states.” During “down-states,” the cortical neurons are hyperpolarized and do not display firing; during “up-states,” they are depolarized as a consequence of the ionic mechanisms described in Chapter 3. In the initial sleep stages (stage 2), spindles at the frequency of about 12 to 15 Hz appear, but these are also present during slow wave sleep (SWS). In the hippocampus sharp wave events combined, or not, with high-frequency ripples occur during SWS and also during waking. Theta oscillations (4 to 8 Hz) are a hallmark of rapid eye movement (REM) sleep and are very prominent in rodents, particularly in the hippocampal formation and associated areas but are less regular in man.
The reasons why it is interesting to discuss EEG slow wave activity (SWA) in the context of the topic of this chapter— Neurocognitive Processes and the EEG/MEG—is that although these EEG slow rhythms are distinctive of sleep, namely SWS and REM stages, sleep plays a most interesting role in memory processes; a second reason is that it appears that infraslow oscillations may modulate behavioral variables also in the awake state. We consider first the association among slow oscillations, sleep, and memory processes, since these are very well documented. Later we describe briefly the modulation of behavioral variables with infraslow oscillations in the awake state.
Indeed certain phases of sleep appear to facilitate the formation, or the retrieval, of memories, indicating one of the most compelling relationship between a dynamical brain state characterized by a low-frequency EEG and a very specific cognitive function: memory. There is ample evidence that shows that sleep plays a critical role in memory consolidation, both explicit and implicit memory (see review in Ref. 52), where consolidation means the postlearning stabilization process that strengthens new memory traces. This was shown for procedural memory tasks (53) and for declarative memory (54). Even an ultrashort period of sleep (a diurnal nap of 6 minutes) is sufficient to enhance declarative memory processing, although an extension of the sleep episode to 35-minute duration further improved memory performance to a significant degree (55).
Notwithstanding the fact that very well conducted investigations yield evidence to support a role of sleep in memory functions, this question has been controversial. For example, Vertes (56) stated that there is no compelling evidence to support a relationship between sleep and memory consolidation, although he admits that memory consolidation initiated in
waking with task acquisition may extend to sleep, but in the same issue of Neuron (2004) dedicated to this theme opposite views were presented (53).
waking with task acquisition may extend to sleep, but in the same issue of Neuron (2004) dedicated to this theme opposite views were presented (53).
The two main stages of sleep, SWS and REM sleep, are characterized by distinct EEG patterns and appear to be associated with different kinds of cognitive processes. SWS has been implicated in the consolidation of hippocampus-dependent, explicit or declarative memory (57,58,78), whereas REM appears to be associated with hippocampus-independent, implicit, procedural, or nondeclarative memory tasks (59).
Memory functions are essential for the survival of animals in general. While the encoding and retrieval of memory are typical properties of the waking state, the distribution of memory traces in the cortex and the consolidation of memory are properties that are strongly influenced by sleep states. Following the analysis of Diekelmann and Born (60), the associations between the two stages of sleep and memory processes can be summarized according to two main concepts:
SWS that comprises the features—slow oscillations, spindles, and ripples—corresponds to a minimal level of cholinergic activity; this neuronal state would coordinate the reactivation and redistribution of hippocampus-dependent memories to the neocortex. Spindle activity and ripples increase during the “up-state” and become suppressed during the “down-state” of the slow oscillation. A current hypothesis is that during the “up-state,” the ripples and spindles would provide a mechanism by which information would be transferred from the hippocampal formation to the neocortex leading to plastic synaptic changes of long duration and the storage of information in the neocortex, as explained in more detail below.
REM characterized by a cortical desynchronized EEG and ponto-geniculate-occipital (PGO) waves concomitant with a regular theta rhythm in the hippocampal formation and associated structures corresponds to a high level of cholinergic activity and a local increase in plasticity-related immediately early genes; this neuronal state would facilitate the subsequent synaptic consolidation of memories in the cortex.
A central question is whether the EEG pattern characteristic of SWS is directly relevant for memory functions or whether it is the duration of sleep as such that is the important variable. The study of van der Werf et al. (61) yields interesting information in this respect. These authors showed that a disruption of sleep that suppressed SWA and induced shallow sleep, but did not reduce total sleep time, was sufficient to affect subsequent successful encoding-related hippocampal activation and memory performance in healthy human subjects. Shallow sleep is characterized by a decrease in SWA (0.5 to 4 Hz) and an increase in alpha (8 to 12 Hz) and higher frequencies. These results indicate that the neuronal processes underlying SWA are important in order to promote memory encoding and subsequent performance. These observations are in line with the experimental findings of Marshall et al. (58) who showed that slow oscillations induced in man by transcranial magnetic stimulation (TMS) during non-REM sleep at low frequency (0.75 Hz) were able to improve the retention of hippocampus-dependent memory, but stimulation at higher frequencies (5 Hz) did not; the low-frequency stimulation, however, was not effective in improving procedural (hippocampus-independent) memory. This suggests a causal role of cortical SWA in some kinds of memory processes. A similar conclusion regarding the role of ripples can be drawn from the findings of Ego-Stengel and Wilson (62) who showed that disrupting selectively neuronal activity during ripple events, by stimulating of hippocampal afferents in the rat, impairs spatial learning.
A subsequent question is which properties of these EEG phenomena, namely the slow waves, are responsible for this effect? A possibility that has been advanced is that the neocortical slow oscillations can induce up- and down-states of neuronal activity throughout the neocortex and subcortical structures and in this way may promote the redistribution of memories for long-term consolidation; this was suggested based on experiments in rodents (63). What about TC spindles? Some investigations both in rat and in man (64,65) found increases in spindle density during non-REM sleep after learning of both declarative tasks and procedural motor skills. It was also shown that these increases in spindle density were correlated with postsleep memory improvement (66) and even appeared to be localized to different cortical areas depending on the kind of memory task. These findings raise the question of what is the basic mechanism by which these sleep phenomena may promote memory. Marshall et al. (58) proposed as a possible mechanism an increase in calcium transients particularly mediated by spindle activity driven by slow oscillations. Indeed Rosanova and Ulrich (67) made in this respect an interesting experiment: they applied a spindle-like stimulation pattern repeated every 1.5 seconds (0.6 Hz) to pyramidal neurons of layer V using whole-cell patch-clamp recordings; in this way, they were able to induce NMDA receptor-dependent short-term potentiation (STP) and long-term potentiation (LTP), which was demonstrated to depend on L-type calcium channels. This finding strongly suggests that this may be one mechanism by means of which this characteristic EEG sleep pattern can specifically induce memory consolidation.
One remarkable link between EEG sleep phenomena and memory is the observation, in rodents that during sleep there is a reactivation or replay, of patterns of activity of assemblies of neurons that were active during memory acquisition in the awake state. Wilson and McNaughton (68) made simultaneous recordings from hippocampal “place cells” during spatial behavioral tasks and in SWS preceding and following these tasks. They found that hippocampal pyramidal cells that had overlapping “place fields” and a correlated firing pattern, preserved the same correlated pattern during subsequent sleep. This correlated activity appears preferentially during hippocampal “sharp waves-ripple complexes” (69). Those authors interpreted this finding as indicating that information acquired in the awake state during active behavior can be re-expressed in hippocampal circuits during sleep (70). Hippocampal sharp waves and the associated high-frequency ripples (˜200 Hz) are temporally correlated with SWS cortical spindles (for a detailed discussion, see Ref. 71, pp. 342-355). Thus, the combination hippocampal “sharp waves-ripples” and “cortical spindles” can form a basic mechanism of
hippocampal-cortical communication during SWS (72). Further reactivation occurs in the neocortex as demonstrated by Ji and Wilson (73) and Peyrache et al. (74) and also in the striatum (75). Furthermore, slow oscillations drive the sharp waves and ripples that are associated with hippocampal reactivation (76); thus, it has been hypothesized that spindle-ripple complexes would form the main mechanism for the transfer of memory traces from the hippocampus to the neocortex (77,78).
hippocampal-cortical communication during SWS (72). Further reactivation occurs in the neocortex as demonstrated by Ji and Wilson (73) and Peyrache et al. (74) and also in the striatum (75). Furthermore, slow oscillations drive the sharp waves and ripples that are associated with hippocampal reactivation (76); thus, it has been hypothesized that spindle-ripple complexes would form the main mechanism for the transfer of memory traces from the hippocampus to the neocortex (77,78).
According to some interpretations, the neocortical circuits, established during SWS, can engage the hippocampus afterward during REM sleep. This reactivation of previous behavioral episode representations may be important for the learning and performance of procedural tasks, such as visual discrimination tasks, that are associated with REM sleep (79).
Is There Experimental Evidence to Allow Extending to Man These Observations Concerning the Redistribution of Memory Traces during SWS Sleep Originally Obtained in Rodents?
Functional brain imaging studies appear to allow this extension to humans. Thus, Gais et al. (80) showed, using functional MRI, that postlearning sleep enhances hippocampal responses during recall of word pairs 48 hours after learning, indicating the engagement of the hippocampal formation in memory processing during sleep, while sleep induces functional connectivity between the hippocampus and the medial prefrontal cortex.
On the contrary during REM sleep, the EEG activity, including theta rhythm, presents reduced coherence between limbic-hippocampal and TC circuits, in comparison both with SWS and wakefulness (81,82). This suggests that distributed memory systems become disengaged during REM, which may protect local processes of synaptic consolidation from interferences and thus may promote consolidation. Whether there are processes characteristic of REM sleep that may specifically promote this memory consolidation is a matter of debate. Both EEG and molecular findings appear to support such an effect. Regarding the former, we should remember that the typical REM sleep cortical EEG is characterized by irregular activity with a predominance of wide-band frequencies, as also seen during waking, but less coherent and practically not disturbed by external inputs. This may represent a state propitious to the reinforcement of local synaptic plasticity. Regarding the latter it has been shown that associated with REM sleep there is an upregulation of plasticity-related immediately early gene activity, which depends on learning during prior wakefulness and appears to be localized in certain brain regions (83). Namely the induction of hippocampal LTP during waking in rats leads to an upregulation of zif-268 gene expression that propagates gradually from the hippocampus to extrahippocampal regions as REM sleep recurs. Furthermore, the expression of these genes is enhanced by cholinergic influences through muscarinic receptors that are also at a high level during REM sleep (84).
Diekelmann and Born (60) remark appropriately that since REM sleep normally follows SWS, it is likely that this sequence may have functional relevance. Along this line of reasoning, they propose the following synthesis: SWS promotes memory at the system level, that is, the distribution of memory traces throughout brain systems, which is followed and complemented by a local synaptic consolidation stage during REM. The quest for the functional relationships between EEG phenomena characteristic of different sleep stages and memory is an exciting field that is in a state of accelerated evolution.
Do the Infraslow Oscillations Also Modulate Cognitive Functions in the Awake State?
At the end of this section, we describe briefly the modulation of behavioral variables with infraslow oscillations in the awake state. In this respect, the study of Monto et al. (85) is particularly interesting. These authors investigated whether the 0.01 to 0.1 Hz infraslow oscillations may influence slow fluctuations in human psychophysical performance. With this purpose, they used an uncued somatosensory detection task; they applied weak electrical pulses to the right index finger, while simultaneously recording the direct current-coupled full-band EEG. The subjects were asked to report a detected stimulus by twitching the right thumb while keeping eyes closed.
The correlations between behavioral performance and infraslow EEG oscillations, recorded at Fpz and Cz, were estimated for both phase and amplitude. The statistical analyses showed that behavioral performance was correlated with the phase of the oscillation, although the reaction times were not affected by this phase. Contrary to the phase, the correlation between the infraslow amplitude and hit probability was very limited at Fpz and absent at Cz.
These findings allow to draw the conclusion that EEG infraslow oscillations are associated with fluctuations of the excitability of cortical networks and can modulate the execution of cognitive tasks.
In conclusion, EEG slow oscillations can modulate cognitive processes, particularly memory formation.
Theta Rhythms, Spatial Cognition, and Memory Processes
Most of the knowledge about the neurophysiology of theta rhythms at the cellular level and at neuronal network levels pertains to the activities recorded in the hippocampal formation and associated limbic structures, including the cingulate cortex, in rodents and other animals (86, see also Chapter 3). The relations between theta, sensory, and motor events is well documented, particularly regarding spatial orientation. Furthermore, the modulation of theta frequency with levels of motor actions (locomotion, orientating, and vestibular stimulation) is also well established in animals. In the case of human theta rhythms one faces, however, two related questions: one is that it is still frequently questioned whether theta rhythms can be recorded from limbic structures in human as in other animals; the other one is whether the theta rhythms recorded from the human scalp are the reflection of hippocampal, or limbic, theta or are locally generated in the cortex. We think that there are now good arguments to reply affirmatively to both questions based mainly on a series of experimental findings as reviewed by Kahana (87).
Are Theta Oscillations Associated with Movement and Spatial Navigation?
A good deal of the human studies that yield insight in the relations between theta rhythms and cognitive processes were obtained using indwelling electrodes, either implanted intracerebrally or incorporated in subdural grids. These recordings permitted the direct exploration of the hippocampal formation in humans during the performance of behavioral tasks. In one of the earlier human studies (88), the hippocampal EEG was recorded using implanted electrodes in a freely moving epileptic patient undergoing deep brain exploration as a part of presurgical evaluation, during a task where the patient heard a word and had to move to a blackboard to write it down. The theta peak frequency (between 3 and 4 Hz) increased as well as the rhythmicity (i.e., bandwidth decreased), with the speed of the patient’s movement, and was highest when the patient wrote the word on the blackboard. This shows that hippocampal low-frequency theta is modulated by overt movement in a situation with a working memory component.
Kahana et al. (89) investigated also epileptic patients during a task in which the subjects had to navigate through a virtual maze. The hippocampal EEG recordings showed periods of theta rhythmic activity as the subjects learned to navigate virtual mazes, and their occurrence covaried with the degree of difficulty of the task suggesting an association between the occurrence of theta oscillations and spatial navigation. These experimental findings were further elaborated by Caplan et al. (90) who showed that theta oscillations appeared more frequently during virtual movement than during periods of voluntary stillness, namely during both searching and goal-seeking behavior. Somewhat surprising, these effects appeared to be widespread, throughout many recording sites including bilaterally the peri-Rolandic region and the temporal lobes. A conclusion drawn from these studies is that theta activity is preeminent during learning of associations between sensory stimuli and motor behavior that are essential for the performance of spatial navigation.
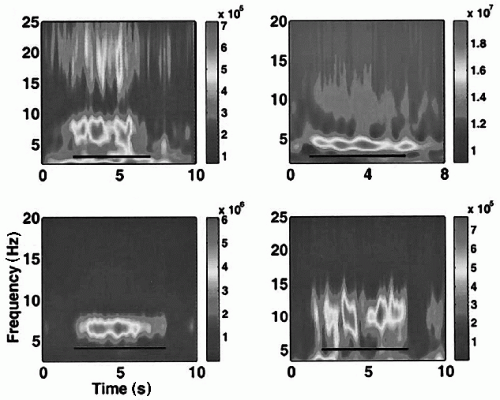 Figure 50.2 Theta is gated during the Sternberg task. Averaged time-frequency power shows sustained theta activity in four subjects: right frontal site in subject 1 (up left); left temporal site in subject 2 (up right); right frontal site in subject 3 (bottom left); depth electrode in left temporal lobe in subject 4 (bottom right). The color scale represents power in square microvolts. (Adapted with permission from Raghavachari S, Kahana MJ, Rizzuto DS, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;121(9):3175-3183.) (See color insert.) |
Are Theta Oscillations Associated with Memory Processes?
A question that was raised in view of these observations is whether the theta oscillations were specifically related to spatial navigation with a strong motor component, or to a learning component that implies a memory function.
This question was tackled by Raghavachari et al. (91) who investigated whether theta occurs during a nonspatial task, namely as subjects performed the Sternberg working memory task (Fig. 50.2). They found that the amplitude of theta rhythmic activity in the range from 4 to 9 Hz, but predominantly between 6 and 8 Hz, increased from the start of a Sternberg task, persisted during the delay period, and decreased only at the end. In this study, the electrode sites where the increases of theta oscillations were observed were also dispersed over many cortical sites (temporal lobe, occipital lobe, parietal/motor/premotor areas, and frontal lobe), although the amount of interareal correlation during the periods of enhanced theta oscillations was not explicitly estimated. Raghavachari et al. (91) concluded that theta oscillations could mediate the organization of working memory, incorporating multiple items of information.
A relevant question that has to be considered is the following: What is the relation between theta oscillations found in the investigations referred to above, that appear in different cortical areas related to memory processes, and that of the hippocampal formation? This question was addressed in several studies where recordings were made from deep structures using indwelling electrodes and from the cortical surface.
Sederberg et al. (92) investigated EEG changes in epileptic patients with intracranial cortical and subcortical electrodes. These authors found predominant theta and gamma oscillations during the encoding stage in a basic memory task. A relationship was observed between increased theta activity in right frontal and temporal regions during encoding and later correct recall although coherence itself was not assessed.
There is evidence in human healthy subjects from studies carried out using scalp EEG that theta oscillations are related to memory processes such as successful encoding of target items (93, 94, 95 and 96) or recognition of previously memorized items (96,97), or episodic memory trace decay, maintenance of items in working memory and task difficulty (98), memory load, involving encoding, maintenance, and retrieval (99). These theta oscillations are induced by the cognitive event and have no strict phase relation with a stimulus. Therefore, these oscillations have to be quantified in the frequency domain by spectral density. An important aspect that is considered only in some studies is how theta oscillations recorded from different areas are interrelated and which are the corresponding sources in the brain. In this respect, Sarnthein et al. (100) found increased 4 to 7 Hz coherence between frontal and posterior association cortex during retention periods involved in a verbal and a visuospatial working memory task. During the verbal task, coherence was stronger between frontal and left posterior regions compared with right posterior regions. During the visuospatial task, coherence with the left posterior region was similar to that noted on the verbal task, while there was greater coherence with right posterior regions. Less coherence was observed during perception intervals when stimuli were presented to the participant. Schack et al. (101) investigated subjects using a modified Sternberg paradigm including either written words or rectangular figures for the stimuli lists and probes. Theta power was found to be maximal at Fz and Cz during retention periods. Furthermore, coherence and strong phase coupling was noted between theta frequencies recorded at Fz and 20 to 30 Hz frequencies recorded at frontal areas (Fp1).
With respect to the relationships between cortical areas during the presence of theta rhythmic activity, Von Stein and Sarnthein (102) proposed that theta oscillations may mediate the integration of neural activities in different cortical areas, as between frontal and parietal cortices. It is not yet demonstrated clearly that these cortico-cortical-coherent neuronal assemblies may also involve the hippocampal formation.
Several studies using MEG have dealt with the same quest for associations between theta activity and memory functions. Tesche and Karhu (103) showed a relation between the duration of episodes of theta increased activity and memory demands in a working memory task, based on an analysis of MEG signals that were interpreted as possibly having their source in the hippocampal formation. Jensen and Tesche (104) recorded whole-head MEG signals while subjects were performing a Sternberg memory task. These authors found that the activity of the band around 8 Hz (called theta by them), recorded particularly from the frontal areas, increased with the number of items retained in working memory. These findings led these authors to conclude that theta activity in frontal brain areas may play a role in memory maintenance. Also using MEG recordings, de Araújo et al. (105) found an oscillation with a peak at approximately 3.7 Hz that increased during a task where subjects had to navigate through computer-generated virtual reality town. Using a single dipole model, they localized this source to near the superior temporal gyrus and the deeper temporal structures. The authors also found that theta activity apparently “propagated” from frontal to posterior regions during the task. The results suggest an association between theta rhythm and the performance of navigational tasks in humans. Guderian and Düzel (106) used whole-head MEG to find out whether MEG phenomena associated with recognition memory judgments could be put in evidence. They showed the subject pictures of human faces, some of which had been seen previously and some not. Theta oscillations, both high (7 to 8 Hz) and low (4.5 Hz), had larger amplitude for those trials where the subjects had recollection in comparison with trials where accurate memory was poor. Using information about the phase differences between sensor pairs at each time point, these authors estimated the brain sources of the theta oscillations. This analysis led to the conclusion that recollection of the memorized information is associated with a distributed network responsible for the generation of theta oscillations, which includes the prefrontal, mediotemporal, and occipital areas. The oscillations would act as a mechanism to bind these cortical areas together during the correct performance of the recollection task. Interestingly, they found also that some cortical areas, namely bilateral prefrontal and left temporal cortices, were activated both in trials where recollection was correct as in those where this was wrong, suggesting that the activity of these cortical areas may be related to the retrieval process, as such, regardless of whether the latter yields correct results or not.
Further Cornwell et al. (107) also using whole-head MEG and a spatial filtering technique (synthetic aperture magnetometry or SAM) reported hippocampal and parahippocampal theta, centered around 5 Hz, during an ingenious spatial navigation task (virtual reality Morris water maze) showing larger theta activity in the left anterior hippocampus specially during the early stages of training, indicating that this region plays a role in early learning.
We have to note that there is a considerable variability among several of these studies with respect to the frequency of theta oscillations that were studied. In several of these investigations, the frequency of the oscillations is relatively high (˜8 Hz) to be assigned without doubt to the hippocampal formation and associated limbic cortex, since the electrical activity recorded locally from electrodes implanted in the human hippocampus (84) is much lower (˜4 Hz), which corresponds close to that reported in de Araújo et al. (105) using MEG.
Furthermore, the properties of the actual behavior of the subject performing a memory task has been addressed by Jacobs et al. (108), who proposed that such correlative studies should preferably take into account multiple behavioral variables involved in the performance of a memory task. The latter study is particularly illuminating in this account because it describes not
only different behavioral variables, but it also takes into consideration different frequency bands, using a multivariate statistical analysis. The task consisted of the presentation of lists of two, four, or six consonants followed by a test probe. The subjects were requested to press a key indicating whether the probe was a target (i.e., an item presented previously) or a lure (i.e., an item not present in the list). The main findings were the following: theta power at left-central electrode sites correlated with the degree of match between the probe and memory contents. In general, theta power correlated with a number of task variables, such as memory recognition (at 300 milliseconds after probe presentation), differentiation between a target and a lure (at 500 milliseconds), reaction time, and memory load, at various latencies after probe presentation. Together with the observation (109) of a preferential distribution of theta power over the left parietal scalp in a task when subjects had to memorize and retrieve open-class words (nouns, verbs, or adjectives) as compared to closed-class words (determiners, conjunctions, or prepositions), we may concur with these authors that left-parietal theta power is correlated, in more general terms, with memory retrieval of lexical-semantic properties of open-class words.
only different behavioral variables, but it also takes into consideration different frequency bands, using a multivariate statistical analysis. The task consisted of the presentation of lists of two, four, or six consonants followed by a test probe. The subjects were requested to press a key indicating whether the probe was a target (i.e., an item presented previously) or a lure (i.e., an item not present in the list). The main findings were the following: theta power at left-central electrode sites correlated with the degree of match between the probe and memory contents. In general, theta power correlated with a number of task variables, such as memory recognition (at 300 milliseconds after probe presentation), differentiation between a target and a lure (at 500 milliseconds), reaction time, and memory load, at various latencies after probe presentation. Together with the observation (109) of a preferential distribution of theta power over the left parietal scalp in a task when subjects had to memorize and retrieve open-class words (nouns, verbs, or adjectives) as compared to closed-class words (determiners, conjunctions, or prepositions), we may concur with these authors that left-parietal theta power is correlated, in more general terms, with memory retrieval of lexical-semantic properties of open-class words.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








