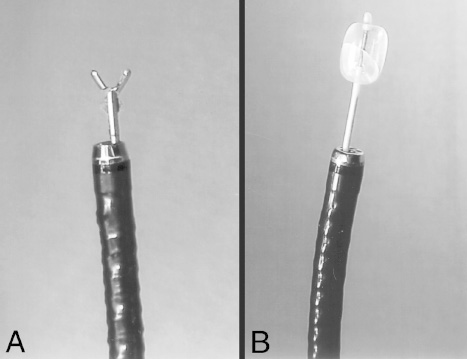
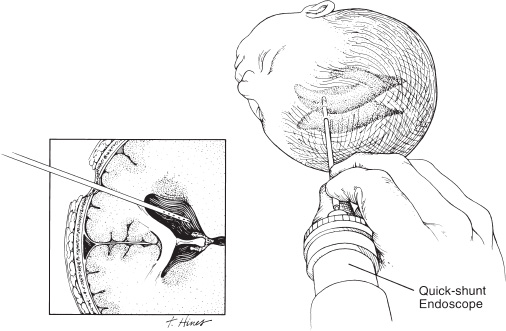
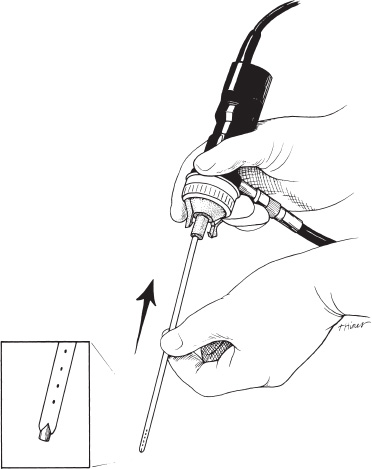
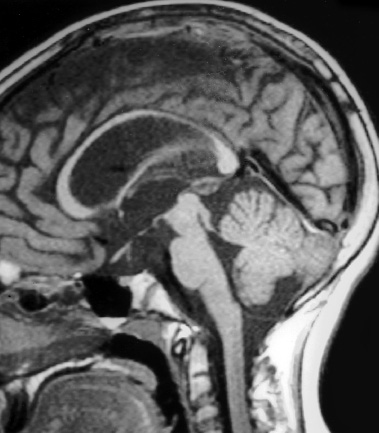
Neuroendoscopy has emerged as a valuable technologic adjunct in the treatment of many neurosurgical conditions. Endoscopically guided shunt placement, membrane fenestration, and tumor management are the most common procedures performed using a neuroendoscope. To date, most neuroendoscopic procedures involve the fenestration of intraventricular cysts or membranes and the biopsy or removal of intraventricular tumors. In most patients, obstructive hydrocephalus accompanies these conditions and produces symptoms of increased intracranial pressure, commonly including headache and vomiting, although accelerated head growth may be the only presenting symptom in neonates or infants. Ultrasound or computed tomography (CT) usually is performed as a screening study to diagnose these conditions. Characteristic images demonstrate hydrocephalus alone or accompanied by intraventricular tumors or cystic membranes. Magnetic resonance imaging (MRI) should be performed prior to any neuroendoscopic procedure because it provides the best anatomic detail of the surrounding vasculature and adjacent brain structures. Preoperative contrast ventriculography should be performed in cases of complex hydrocephalus to evaluate communication between compartments within the ventricular system. No additional diagnostic studies are needed prior to neuroendoscopic surgery. Essential components for performing surgery include an endoscope, either rigid or flexible, light source, camera, viewing monitor, irrigation pump, and instrumentation for dissection and coagulation. Typically, the components are assembled on a wheeled cart, or they may be manufactured in a unitized system. Rigid endoscopes have traditionally been favored over fiberscopes because of their superior optics and multiple ports for irrigation and suction. Rigid endoscopes are difficult to maneuver through the ventricles when performing multiple fenestrations and require strategic targeting of the initial burr hole. Fiberscopes provide greater maneuverability with less strategic planning but compromise optical clarity and image quality. In many procedures, both types of endoscope may be used. Although the instrumentation for performing endoscopic surgery is limited, specific instruments are available to perform biopsy, to dissect, and to coagulate tissue. Cup forceps are essential for tissue biopsy and for grasping foreign bodies within the ventricular system (Fig. 2–1A). Small balloon catheters are available to enlarge small fenestrations created in the septum pellucidum, the floor of the third ventricle, or walls of obstructing cysts (Fig. 2–1B). Fiberoptic lasers and monopolar electrodes may be used as energy sources to coagulate tumors or membranes and to dissect tissue. Both have been used successfully, but the higher cost of laser fibers and the concern about heat dissipation from the laser have favored the emergence of a monopolar electrode as the preferred device in neuroendoscopic surgery. Additional instrumentation consisting of scissors, probes, and various configured forceps are available, but they have limited use and are awkward to use. Bipolar devices have been manufactured, but they depart from the traditional design used in conventional neurosurgery, giving them limited benefit. FIGURE 2–1. A: Cup forceps used for biopsy. B: Inflated balloon catheter used to enlarge fenestrations. The most important factor in planning an endoscopic procedure is the location of the burr hole. For lesions in the anterior half of the ventricular system, the burr hole should be created anterior to the coronal suture. For lesions in the posterior half of the ventricular system or temporal horn, the burr hole should be positioned at the parietal eminence (Fig. 2–2). Navigational guidance systems may be necessary when the ventricles are small or when the targeted lesion requires a precise point for entering the ventricular system or cerebral parenchyma. Patient position becomes important once the entry point has been selected. A supine position is optimal for lesions in the anterior half of the ventricles, whereas a prone position is necessary when entering the posterior part of the ventricular system or the posterior fossa. The head is usually placed in a cerebellar or soft doughnut headrest when the patient is positioned supine. If the patient is placed in the prone position, a cerebellar headrest is used. Three-point cranial fixation is rarely necessary unless an open craniotomy is planned and the endoscope is to be used as an adjunct surgical device. Medications recommended for neuroendoscopic procedures include prophylactic antibiotics and intravenous steroids. Arterial lines, large-bore intravenous lines, and urinary catheters are seldom necessary in endoscopic procedures. Following routine intubation, a balanced anesthetic technique consisting of an inhalational agent and short-acting narcotic is used. The patient is mildly hyperventilated until the ventricle is cannulated. When the endoscope is introduced, homeothermic lactated Ringer’s solution is used for irrigation. Ringer’s solution is preferred over NaCl 0.9% because it is nearer the normal physiologic composition of cerebrospinal fluid. FIGURE 2–2. Patient position demonstrating entry sites for the coronal and parietooccipital approaches. (Printed with permission from Mayfield Clinic.) Creation of a ventriculoperitoneal shunt is a common procedure in pediatric neurosurgery. There is considerable controversy regarding the optimal position for entering the ventricular system. My preference is to place the ventricular catheter from the frontal or coronal approach. A small pen neuroendoscope may be used for optimal positioning of the intraventricular catheter. The patient is positioned supine on the operating table with a small roll placed beneath the shoulders to elevate the chest. The head is turned 90 degrees to the left side so that the nasal–occipital line is parallel to the floor. A small doughnut roll is placed beneath the head. If shaving the hair is the surgeon’s preference, the right frontal region and a small area of the right parietal region may be shaved clean of hair for a frontal approach. A standard surgical preparation of the skin is used, followed by draping the field with sterile sheets. Skin incisions are infiltrated with a preparation of lidocaine and epinephrine. A small curvilinear incision is made in the right frontal region. The burr hole is created with a highspeed drill sufficiently large to accommodate the reservoir of the intraventricular catheter. An integrated valve with distal catheter is tunneled beneath the skin first to the parietal incision and then to the abdomen. The dura mater is incised and the arachnoidal membrane is coagulated with bipolar cautery. The neuroendoscope is inserted into the proximal catheter and passed through the brain into the lateral ventricle (Fig. 2–3). The neuroendoscope then is passed through a precut slit in the ventricular catheter to view the ventricular system (Fig. 2–4). Normal ventricular landmarks should be identified for proper orientation. Once the surgeon feels familiar with the orientation, the catheter with the endoscope is moved in unison toward the foramen of Monro. Any abnormal findings should be noted and may include deposits of hemosiderin from a previous hemorrhage, thickening of the ependyma from previous infection, or thin transparent cystic lesions not identified on radiographic imaging. The association of these findings with shunt malfunction is being investigated. To date, patients with dense hemosiderin deposits appear to experience early malfunction, most likely related to free-floating fragments obstructing the proximal catheter or valve. Patients found to have membranes may demonstrate ventricular loculations. After the tip of the endoscope is navigated to the choroid plexus, the proximal catheter is deposited to rest just above this near-contact point. The endoscope is withdrawn and the catheter is attached to the reservoir and valve with 3–0 silk ligatures. Cerebrospinal fluid flow is observed from the distal end of the shunt before the distal catheter is inserted into the abdominal cavity. After the reservoir and valve assembly are secured to the pericranium with 3–0 silk suture, the skin incisions are closed in an anatomic multiple-layer fashion. FIGURE 2–3. Ventricular catheter inserted into the lateral ventricle. The quick-shunt endoscope substitutes as a stylet for the ventricular catheter. Inset: Coronal view shows correct placement of the catheter and stylet. (Printed with permission from Mayfield Clinic.) Endoscopic third ventriculostomy has attracted the most attention of all neuroendoscopic procedures. The ability to treat obstructive hydrocephalus without the insertion of a diversionary shunt is appealing to the surgeon, patient, and parents. Despite the current interest in third ventriculostomy, the long-term patency of the fenestration has not been clearly established. In 1923, Mixter performed the first endoscopic third ventriculostomy. Additional reports, including many recent publications, showed early high patency rates, but the long-term success of third ventriculostomy is uncertain. Ideal candidates for third ventriculostomy are school-age children or young adults with late-onset hydrocephalus from aqueductal stenosis. Success rates in these groups have been reported to be as high as 85%. Less success is seen in obstructive hydrocephalus resulting from hemorrhage or infection, but neither condition is a contraindication to performing endoscopic third ventriculostomy. It is essential to perform MRI in the preoperative planning for third ventriculostomy because the size of the third ventricle and massa intermedia and the position of the basilar artery are best demonstrated by MRI (Fig. 2–5). The third ventricle should measure at least 3 to 4 mm to accommodate the endoscope, and the floor should not be obstructed by an enlarged massa intermedia. The basilar artery lies beneath the mammillary bodies, leaving a corridor for the fenestration between these structures and the more anterior infundibular recess. The patient is positioned supine on the operating room bed with the head in 0-degree rotation. Following the administration of general anesthesia, the scalp is prepared in the usual fashion and draped with sterile sheets. Following infiltration of the skin with lidocaine and epinephrine, a 2-cm linear incision is made 2.5 to 3.0 cm from the midline just anterior to the coronal suture. The coronal suture is identified, and a 1-cm diameter burr hole is created (Fig. 2–6). The dura is incised and coagulated. A peel-away sheath introducer is guided into the ventricular system. The stylet is removed, and the endoscope is inserted through the sheath into the lateral ventricle. After the proper orientation is achieved, the lateral ventricle should be inspected and the foramen of Monro identified (Fig. 2–7). The endoscope is advanced into the third ventricle, and the floor of the third ventricle is inspected to identify the infundibular recess anteriorly and the mammillary bodies posteriorly (Fig. 2–8A). If a steerable fiberscope is used, the tip of the endoscope may be maneuvered posteriorly to inspect the aqueduct of Sylvius (Fig. 2–8B). A stenotic aqueduct will appear small compared with a normally sized aqueduct (Fig. 2–9A-B).
OVERVIEW
SURGICAL INDICATIONS
INSTRUMENTATION
INTRAOPERATIVE TECHNIQUES
Shunt Placement
Third Ventriculostomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


