3
Neuroimaging of the Posterior Fossa
Neuroimaging of the posterior fossa is a diagnostic challenge for neuroradiology, radiology, neurology, and neurosurgery.1–5 Due to the dramatic technical progress that has been made in imaging modalities, clinicians must be knowledgeable about the imaging techniques and the indications for determining when these examinations are necessary. This chapter provides an overview of the present use and interpretation of imaging modalities for the posterior fossa. A discussion of the physical and technical basic principles of the imaging modalities is beyond the scope of this chapter. But a brief summary of the different imaging techniques, their indications, and the major advantage of focused techniques are presented here, along with the anatomic details of the posterior fossa and some historical background information.6–13 Radiologic cases are presented that demonstrate subtle pathologic findings, and interlinking the different modalities of neuroimaging can confirm the diagnosis.
♦ Topographic Anatomic Details of the Posterior Fossa
The posterior cranial fossa is the deepest and most capacious of the three cranial fossae. It contains the cerebellum, pons, and medulla oblongata. The great foramen is centrally located in the posterior fossa. The posterior fossa is surrounded by deep grooves containing the transverse and sigmoid sinuses (Fig. 3.1). Neuroimaging can portray this detailed neuroanatomy to assist in evaluating the posterior fossa.
The brainstem is formed by the midbrain, pons, and medulla oblongata, and is partially obscured by the cerebral hemispheres and cerebellum. Small motor or sensory nuclei of the cranial nerves are scattered in the gray matter in the brainstem. The midbrain is located between the pons and the cerebral hemispheres. The dorsal segment of the midbrain is called the tectum, and the more central and ventral part is called the tegmentum. The pons lies anterior to the cerebellum and superior to the medulla, from which it is separated by a groove through which the abducens and the facial and acoustic nerves emerge. The medulla oblongata is the pyramid-shaped segment of the brainstem between the spinal cord and the pons. The lower half contains the remnants of the central canal. The posterior portion of the superior half forms the floor of the body of the fourth ventricle. The cerebellum is located in the posterior fossa of the skull, behind the pons and the medulla. It is separated from the overlying cerebrum by an extension of dura mater and the tentorium of the cerebellum. The tentorium of the cerebellum is oval in form, with its widest diameter along the transverse axis. It is composed of a small, unpaired central portion, called the vermis, and two large lateral masses—the cerebellar hemispheres (Fig. 3.2).
♦ Imaging Modalities for the Diagnosis of the Posterior Fossa
The imaging modalities can be divided into noninvasive and invasive, as discussed in the following subsections.
Ultrasound
Since its introduction in the late 1950s, ultrasonography has become a very useful prenatal diagnostic tool in obstetrics and gynecology. The ultrasound scan is currently considered to be a safe, noninvasive, accurate, and cost-effective examination of the fetus. Many structural abnormalities in the fetus can be reliably diagnosed by an ultrasound scan, and these scans can usually be performed before the 20th gestational week. Common examples of neurologic malformations include hydrocephalus, anencephaly, and myelomeningocele. Ultrasound scan will be supplemented with Doppler ultrasound, and the three-dimensional (3D) and four-dimensional (4D) ultrasound techniques.
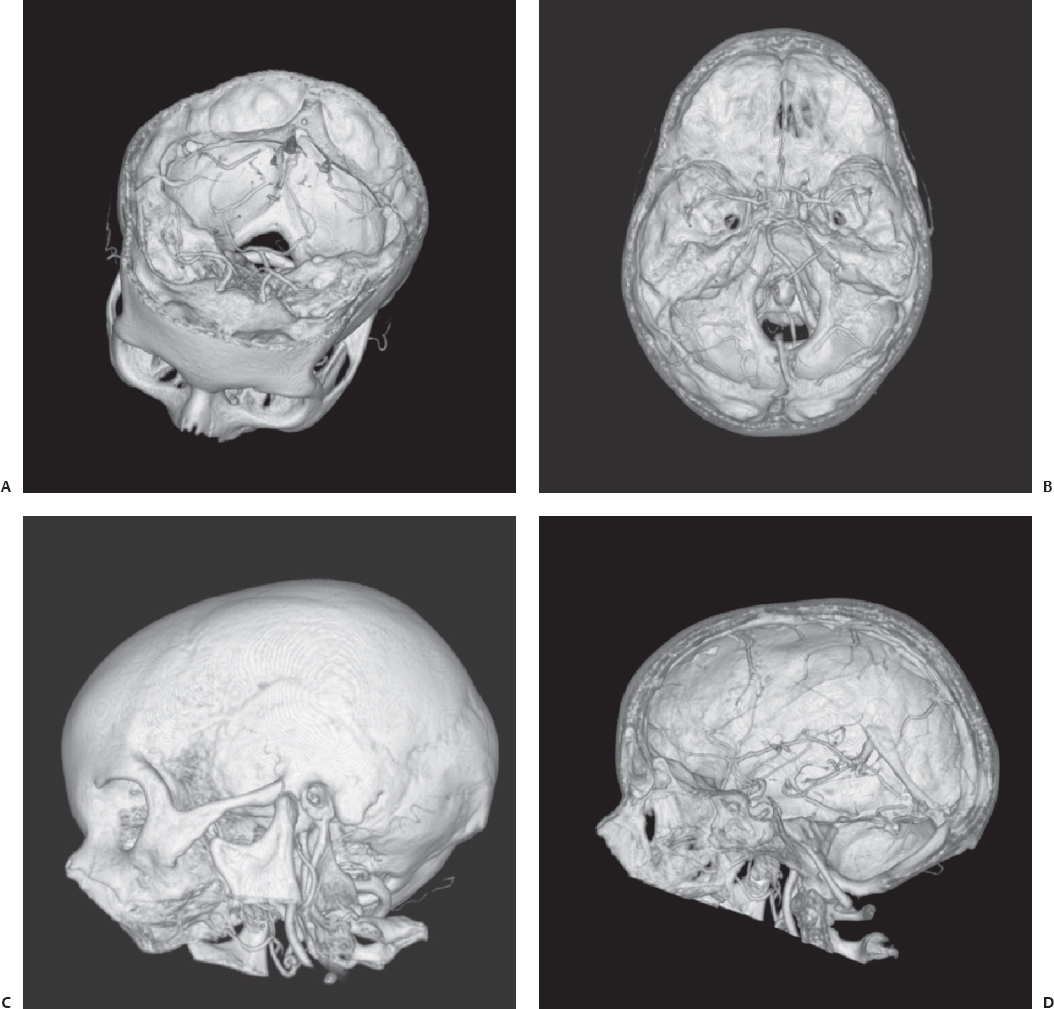
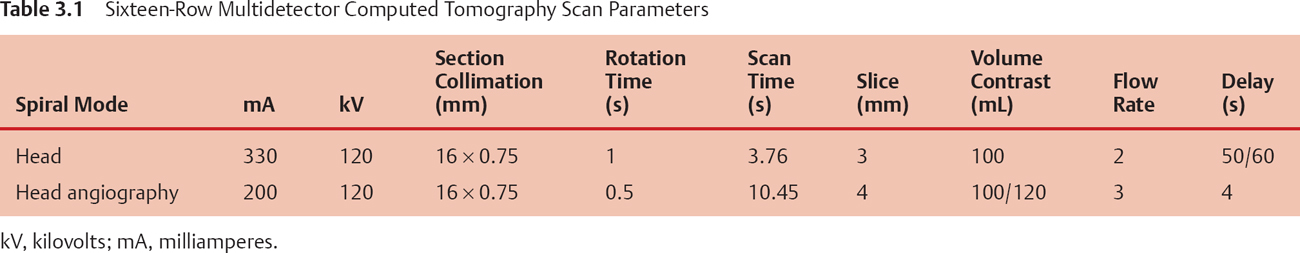
Fig. 3.1 (A–D) Three-dimensional (3D) volume-rendering (VR) scan images of the skull base processed with 3D volume data of a 16-row multidetector CT (MDCT).
Plain X-Ray of the Skull
The oldest modality of imaging is a plain skull x-ray. This technique can show abnormalities and fracture of the skull, signs of chronic intracranial hypertension, and calcification. In cases of a dermoid cyst, a bone defect with sclerotic margins may be detected. But this technique is limited to direct or indirect bone lesions (Fig. 3.3).
Computed Tomography Scanning
Since 1976 examinations of the head have been performed with computed tomography (CT). As CT scan times have gotten faster, more anatomy can be scanned in less time. With an older CT scanner, the examination of the posterior fossa was limited because of the artifact produced from the surrounding thick bone. However, with the new technique of multidetector (4-row, 16-row) CT scanners, high-quality images can be reconstructed in multiple planes from a single volume data set (Fig. 3.4). Multidetector CT (MDCT) is rapidly becoming the new standard in radiologic imaging (Table 3.1).
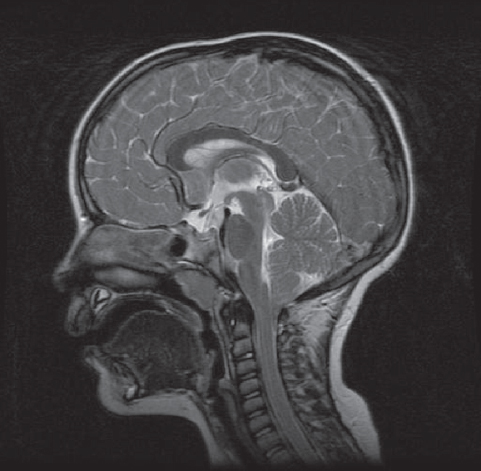
Fig. 3.2 Sagittal view of a T2-weighted magnetic resonance imaging (MRI) scan of the head of an 8-year-old boy. The indication for MRI examination was a continuous headache after the boy fell from his bicycle. The diagnosis was Chiari I malformation with herniation of the cerebellar tonsils through the great foramen into the cervical spinal canal.
The most important primary indications for CT imaging, including CT angiography and CT venography, in neuroradiology are acute head trauma, suspicion of acute intracranial hemorrhage, immediate postoperative evaluation for surgical treatment, shunted hydrocephalus, brain herniation, suspected mass or tumor, and acute cerebral infarction (Fig. 3.5).
Usually, CT is the imaging method of choice in patients with posterior fossa masses who often present with nausea, vomiting, ataxia, and other signs of increased intracranial pressure. CT is a quick, available, and relatively inexpensive method to assess neurologic emergencies including hydrocephalus, hemorrhage, and herniation syndromes.
Magnetic Resonance Imaging Scanning
In the early 1980s, magnetic resonance imaging (MRI) caught the attention of clinicians because of its ability to visualize abnormalities in the posterior fossa of the brain and in the upper cervical spine. Since its clinical development, the application of MRI sequences has rapidly evolved. New techniques and sequences are constantly being developed. To select the right technique, an understanding of the relationships between the various imaging parameters is necessary. With all these techniques, it is evident that MRI is not a modality in which pathology will show itself. The imaging parameters need to be modified to yield the optimum contrast for the particular pathology under investigation (Table 3.2).
Fig. 3.3 This figure demonstrates a fracture of the occipital bone on the right side in a 25-year-old man who arrived at the hospital unconscious and inebriated. (A) The injury is demonstrated in a conventional frontal plain film of the head. On the plain skull x-ray this fracture can be seen in projection at the right frontal sinus. (B) Three-dimensional VR scan images of the skull base, shows the fracture is in the occipital bone.
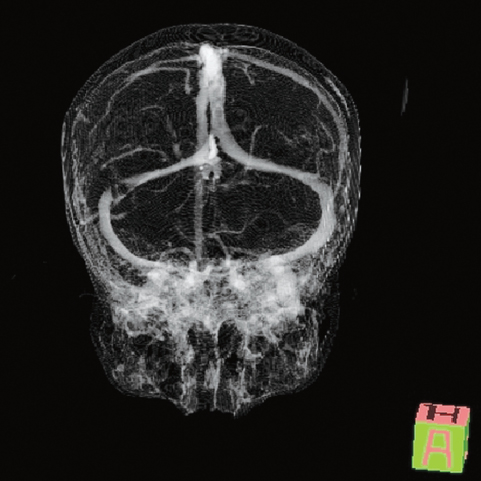
Fig. 3.4 Thrombosis in the right transverse sinus. This image is reconstructed with the same data set as the images in Fig. 3.1.
Furthermore, intrauterine MRI facilitates subtle prenatal neurologic diagnosis. The most important primary indications for MRI in neuroradiology are congenital disorders, posterior fossa lesions, early infarction, demyelinating and other white matter diseases, sensorineural deafness, and inflammatory lesions. The technical parameters of MRI examination vary with the presenting indication (Table 3.3).
Diagnostic Cerebral Angiography
In searching for an intracerebral aneurysm, the second diagnostic procedure to be performed immediately after emergency CT is selective cerebral angiography. Both the carotid and the vertebral arteries must be injected. External carotid arteries should also be visualized, particularly if intracranial angiography is negative, as subarachnoid hemorrhage may sometimes be due to a rupture not of an aneurysm but of a dural arteriovenous malformation. Angiography should aim at recognizing the aneurysm, its precise location, its size, the size of the neck, the relationship with the parent vessel, and its multiplicity. To achieve this goal, the ideal procedure is rotational angiography with 3D reconstruction.
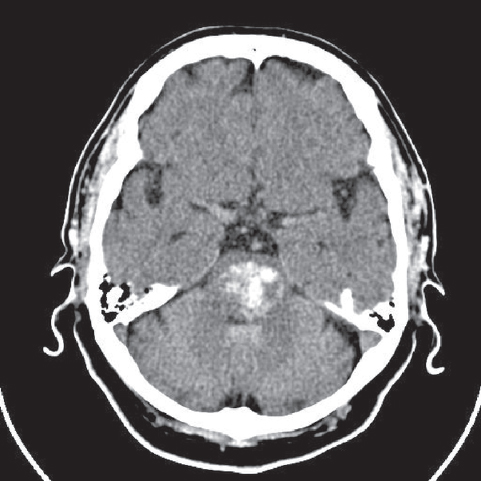
Fig. 3.5 A CT scan of a brainstem hemorrhage (bleeding into the pons).
Furthermore, cerebral angiography is useful in assessing the vascular supply of the tumor. With the wide availability of MRI, cerebral angiography is no longer the first option in brain tumor assessment.
The risks of cerebral angiography are bleeding at the site of the catheter insertion, allergic reaction to x-ray contrast (approximately 1/50,000 to 1/150,000 people), arterial embolism, and stroke.
Examples of pathologic findings in the posterior fossa are presented in Figs. 3.6, 3.7, 3.8, 3.9, 3.10, 3.11, 3.12, and 3.13.
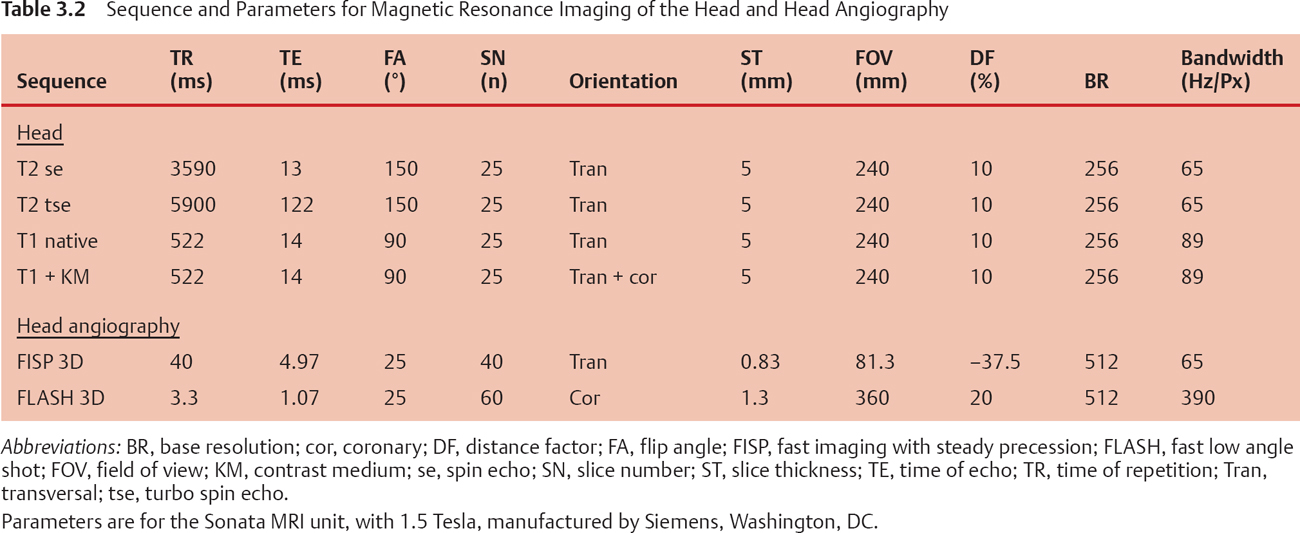
Table 3.3 Common Lesions of the Posterior Fossa
| Child | Adult | |
|---|---|---|
| Extraaxial | Dandy-Walker malformations | Schwannoma |
| Epidermoid cyst | Meningioma | |
| Arachnoid cyst | Epidermoid cyst | |
| Rhabdoid cyst | Aneurysm | |
| Metastasis | ||
| Arachnoid cyst | ||
| Petrous lesions | ||
| Intraaxial | PNET (medulloblastoma) | Infarction |
| Pilocytic astrocytoma | Metastasis | |
| Ependymoma | Hemangioblastoma | |
| Pontine astrocytoma | Multiple sclerosis | |
| Abscess | ||
| Osmotic myelinolysis | ||
| Lhermitte-Duclos sign |
Abbreviation: PNET, permeative neuroectodermal tumor.
From Smirniotopoulos JG. World Health Organization Classification of Brain (CNS) Tumors. 2000 http://rad.usuhs.mil/rad/who/who-index.html.
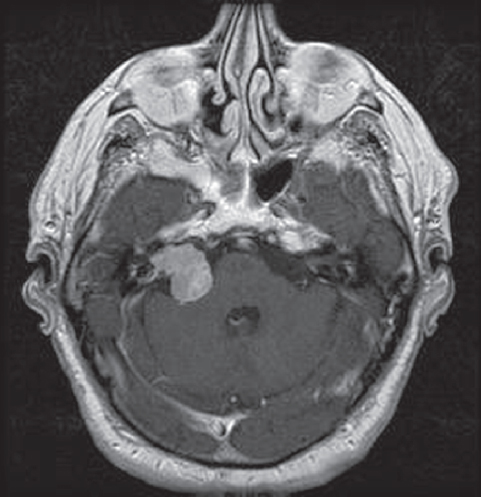
Fig. 3.6 Postcontrast axial T1-weighted MRI. At the right cerebello-pontine angle a contrast-enhanced acoustic neurinoma is located.
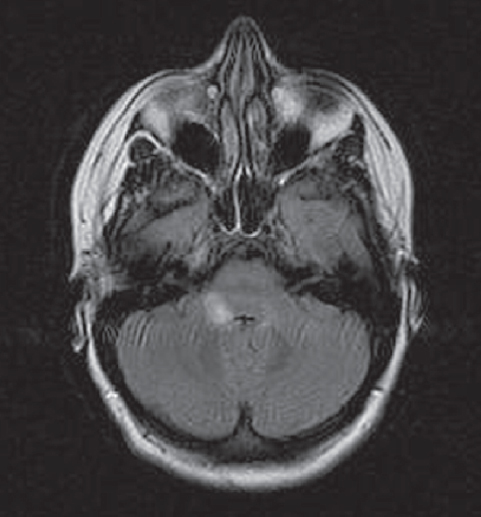
Fig. 3.7 Multiple sclerosis with lesions in the posterior fossa at the right cerebellopontine angle.
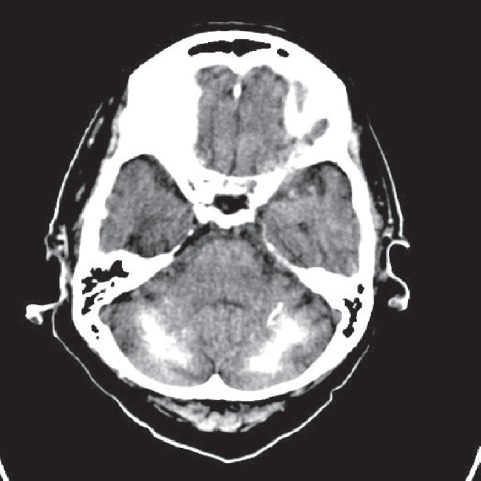
Fig. 3.8 Noncontrast CT shows bilateral symmetric calcification of the cerebellar hemispheres that could be idiopathic, familial cerebrovascular ferrocalcinosis, postinflammatory, congenital, or postanoxic/toxic.
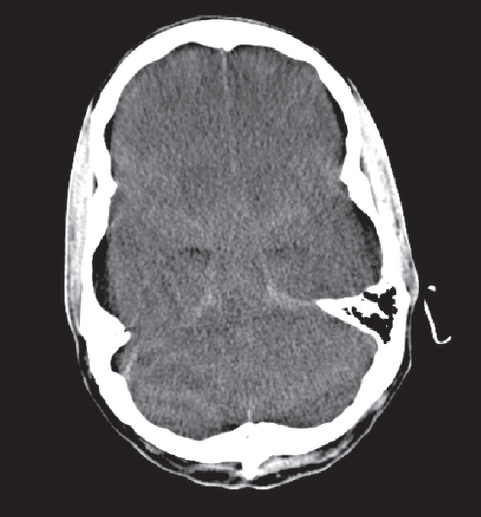
Fig. 3.9 Edema of the cerebrum and cerebellum with compression of the mesencephalon by the tentorium of the cerebellum.
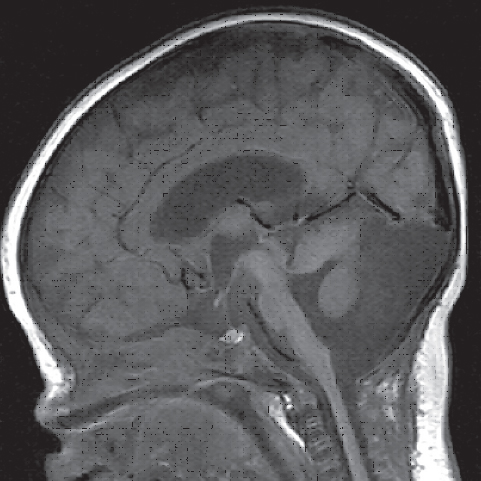
Fig. 3.10 Dandy-Walker malformation with these key features: large posterior fossa cyst, elevated confluence of the sinus (torcular Herophili), hypoplastic vermis, and hypoplastic cerebellar hemispheres.
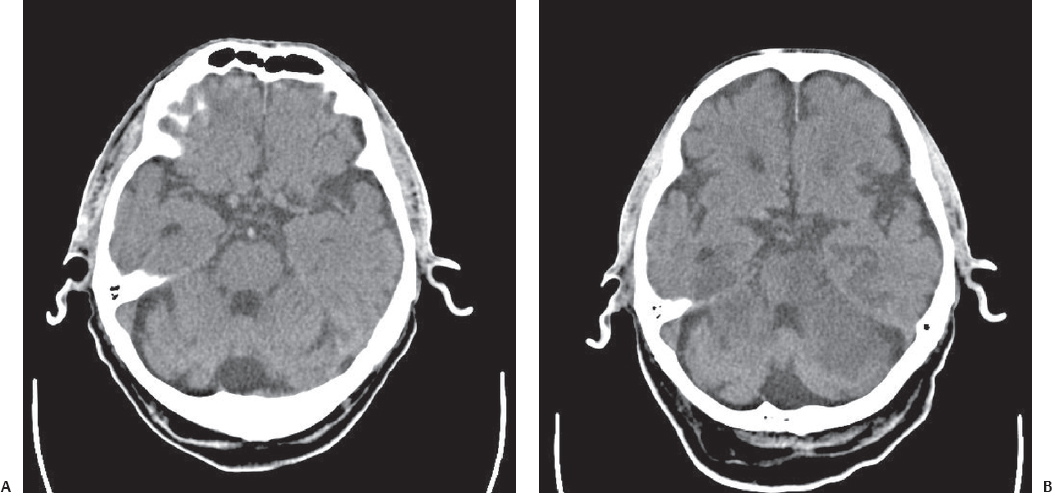
Fig. 3.11 Thrombosis of the basilar artery (A) with an infarction area developing in 12 hours (B).
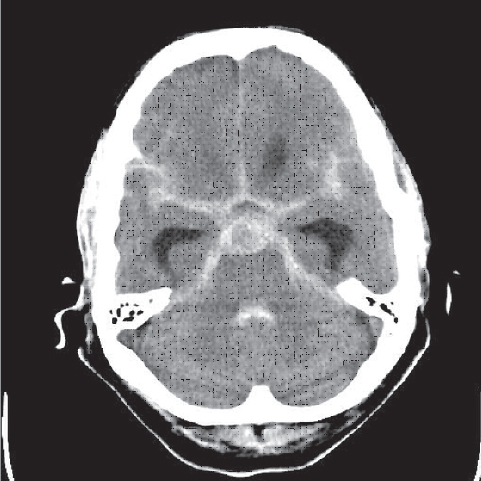
Fig. 3.12 Ruptured aneurysm of the basilar artery.
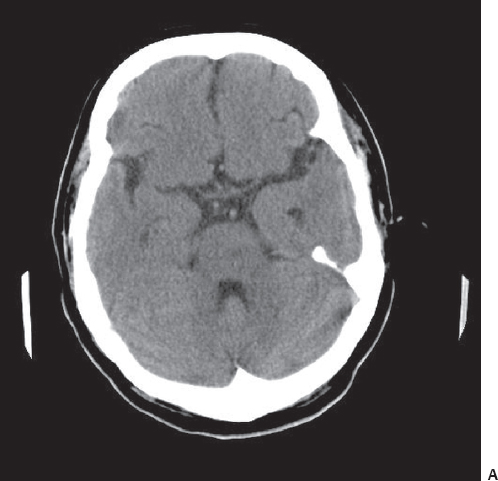
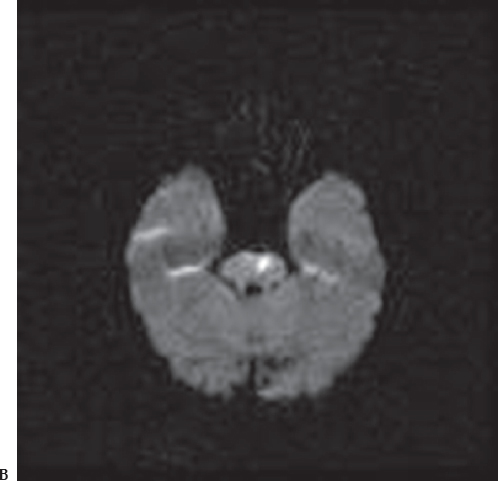
Fig. 3.13 CT scan (A) show a subtle hypodense area in the pons at the left side, whereas diffusion MRI scan (B) detects ischemia in this region with high signal intensity.
References
1. List of ACR Practice Guidelines and Technical Standards. http://www.acr.org/departments/stand_accred/standards/dl_list.html
2. RCR Guidelines for Head Imaging. http://www.rlbuht.nhs.uk/content/pol.asp?web=72&sub=124&page=331
3. Provenzale JM. Centennial dissertation. Honoring Arthur W. Good-speed, MD and James B. Bullitt, MD. CT and MR imaging and nontraumatic neurologic emergencies. AJR Am J Roentgenol 2000;174:289–299 PubMed
4. Luna JA, Goldstein RB. Sonographic visualization of neonatal posterior fossa abnormalities through the posterolateral fontanelle. AJR Am J Roentgenol 2000;174:561–567 PubMed
5. Smirniotopoulos JG. World Health Organization Classification of Brain (CNS) Tumors. 2000 http://rad.usuhs.mil/rad/who/who-index.html
6. Tatter SB. The New WHO Classification of Tumors Affecting the Central Nervous System. http://neurosurgery.mgh.harvard.edu/newwhobt.htm
7. Albright L. Posterior fossa tumors. Neurosurg Clin N Am 1992;3:881–891 PubMed
8. Fukui MB, Hogg JP, Martinez AJ. Extraaxial ependymoma of the posterior fossa. AJNR Am J Neuroradiol 1997;18:1179–1181 PubMed
9. Eslick GD, Hammond SR. Cystic lesion of the posterior fossa. Lancet 2002;359:396 PubMed
10. Maeta M, Saito R, Nameki H. False-positive magnetic resonance image in the diagnosis of small acoustic neuroma. J Laryngol Otol 2001;115: 842–844 PubMed
11. Mangels KJ, Tulipan N, Tsao LY, Alarcon J, Bruner JP. Fetal MRI in the evaluation of intrauterine myelomeningocele. Pediatr Neurosurg 2000; 32:124–131 PubMed
12. Loevner LA. Imaging features of posterior fossa neoplasms in children and adults. Semin Roentgenol 1999;34:84–101 PubMed
13. Tan TY, Teh HS. Contrast-enhanced magnetic resonance imaging of the internal auditory canals and posterior fossa. Ann Acad Med Singapore 1998;27:168–172 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree