Neurologic Disease during Pregnancy
Alison M. Pack
INTRODUCTION
Pregnancy and the postpartum period are times of major biologic and social changes. Pregnancy may be associated with alterations in preexisting neurologic conditions, such as epilepsy or migraine, or herald the emergence of neurologic disorders such as peripheral nerve entrapment or a movement disorder. This chapter addresses the diagnosis, management, and treatment of neurologic disorders arising in or altered by pregnancy.
BIOLOGY OF PREGNANCY
Physiologic changes during pregnancy may influence the expression of neurologic disease and complicate management. Alterations in neuroactive steroid hormones may influence the phenotypic appearance of the disease. Changes in pharmacokinetics, medication compliance, and sleep patterns may make disease management more challenging.
The concentration and type of circulating steroid hormones change during pregnancy. Estrogen production increases. In the nonpregnant state, the main circulating estrogens are estradiol, which is synthesized by ovarian thecal cells, and estrone, which is produced by the extraglandular conversion of androstenedione. Estriol is a peripheral metabolite of estrone and estradiol. In pregnancy, the concentrations of all these estrogens, particularly estriol, increase. As pregnancy progresses, maternal steroids and dihydroisoandrostene from developing fetal adrenal glands are converted principally to estriol. Progesterone production also increases dramatically. These hormonal changes may affect neurologic conditions that are hormone responsive, including migraine, epilepsy, and multiple sclerosis (MS).
Drug pharmacokinetics is affected by the physiologic changes of pregnancy (Table 124.1). Renal blood flow and glomerular filtration increase as a function of increased cardiac output. Plasma volume, extravascular fluid, and adipose tissue increase to create a larger volume of distribution. Serum albumin decreases, which reduces drug binding, increases the free fraction, and increases drug clearance. These pharmacokinetic alterations may affect drug concentrations and are most important for drugs that are highly protein bound, hepatically metabolized, or renally cleared.
TABLE 124.1 Physiologic Changes during Pregnancy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Other events of pregnancy that may compromise management are hyperemesis gravidarum, sleep deprivation, and poor compliance. Hyperemesis gravidarum can make it difficult to maintain adequate concentrations of oral medications. Sleep deprivation aggravates many neurologic conditions and can be a particular problem in the third trimester. Compliance may deteriorate because of a woman’s concern that taking medication might harm her baby. Women are often advised by friends, relatives, and even medical personnel to minimize fetal drug exposure. This may lead to skipped doses, reduced doses, or even self-discontinuation of an indicated medication.
EPILEPSY
Each year, 20,000 women with epilepsy become pregnant. This number has grown as marriage rates have increased for women with epilepsy, as parenting has become more socially supported, and as the medical management of pregnancy in women with epilepsy has improved.
Although most women do maintain good seizure control throughout pregnancy, particularly if they have been seizure free for the 9 months preceding conception, seizure frequency may change. Changes responsible for this include changes in sex hormones, antiepileptic drug (AED) metabolism, sleep schedules, and medication compliance. AED concentrations may change. The total AED concentration falls because of an increase in volume of distribution, decreased drug absorption, and increased drug clearance. Lamotrigine (Lamictal) concentrations decrease throughout pregnancy because of markedly increased clearance. Monitoring of this medication should be performed at least monthly and appropriate adjustments made. Concentrations of levetiracetam (Keppra), oxcarbazepine (Trileptal), and topiramate (Topamax) also decrease in pregnancy and doses need to be adjusted. For some AEDs that are highly protein bound, although the total concentration decreases, the proportion of unbound or free drug increases because albumin levels and protein binding decline. Therefore, it is necessary to follow the non-protein-bound drug concentrations for AEDs that are highly protein bound, including carbamazepine (Tegretol), phenytoin sodium (Dilantin), and sodium valproate (Depakene). Dose adjustments should maintain a stable non-protein-bound fraction.
TERATOGENICITY
The incidence of major congenital malformations (MCMs) in association with in utero AED exposure is increased at least twoor threefold when compared to the general population. Current published risks are derived from data gathered from international prospective registries. A prospective U.S.-based North American
registry continues to gather information about pregnancy and fetal outcome in women using AEDs. This registry should be contacted regarding any woman who becomes pregnant while taking AEDs (1-888-233-2334). A European registry includes countries throughout Europe, Asia, and the continent of Australia and prospectively records information about the effects of AEDs as monotherapy on the developing fetus. The Australian group has independently published their findings. A U.K. register is also actively following pregnant women with epilepsy. MCMs related to AED exposure include cleft lip and palate, cardiac defects (atrial septal defect, tetralogy of Fallot, ventricular septal defect, coarctation of the aorta, patent ductus arteriosus, and pulmonary stenosis), and urogenital defects.
registry continues to gather information about pregnancy and fetal outcome in women using AEDs. This registry should be contacted regarding any woman who becomes pregnant while taking AEDs (1-888-233-2334). A European registry includes countries throughout Europe, Asia, and the continent of Australia and prospectively records information about the effects of AEDs as monotherapy on the developing fetus. The Australian group has independently published their findings. A U.K. register is also actively following pregnant women with epilepsy. MCMs related to AED exposure include cleft lip and palate, cardiac defects (atrial septal defect, tetralogy of Fallot, ventricular septal defect, coarctation of the aorta, patent ductus arteriosus, and pulmonary stenosis), and urogenital defects.
Data is available for many of the commonly used AEDs. Among the currently available AEDs, valproate is consistently associated with the highest rate of MCMs including a higher risk of neural tube defects. When compared to other AEDs, the relative risk is threefold and the absolute risk is 6% to 9% of exposed pregnancies. Results from the European Surveillance of Congenital Anomalies (EUROCAT) found that in utero valproate exposure resulted in a 12.7-fold increase (odds ratio [OR] 12.7, 95% confidence interval [CI] 7.7 to 20.7) of spina bifida when compared to those with no exposure. Findings suggest the risk of valproate-associated MCMs increases at doses above 700 mg/day. Phenobarbital, which is used throughout the world, particularly in developing countries, results in a significantly increased risk of MCMs, notably cardiac malformations. First-trimester topiramate exposure is associated with an increased risk of oral clefts. The rate of oral clefts in the North American Registry was 1.4% (approximately 10-fold higher than control prevalence of 0.11%) and the U.K. Register was 2.2% (U.K. population prevalence is 0.2%). Although carbamazepine has a lower total risk of MCMs (2.6% to 3.8%) than valproate, results from EUROCAT found a 2.6-fold increased risk of spina bifida (OR 2.6, 95% CI 1.2 to 5.3) when compared to those with no exposure. The range of MCMs for lamotrigine in different studies is between 2.0% and 4.6%. Emerging data are encouraging for levetiracetam (Keppra) with published rates of MCMs between 0.7% and 2.4%. Data is limited for other commonly used AEDs including gabapentin, lacosamide, oxcarbazepine, pregabalin, and zonisamide.
Polytherapy is an independent risk factor for teratogenicity. Rates of major malformations are elevated in children exposed to multiple AEDs. Studies find that the children of pregnant women who took multiple AEDs have a higher rate of teratogenicity.
Several mechanisms have been postulated to explain the teratogenicity of AEDs. Some AEDs may be teratogenic because of free radical intermediates that bind with RNA and disrupt DNA synthesis and organogenesis. Higher concentrations of oxide metabolites increase the risk of fetal malformations. Some AEDs cause folic acid deficiency, which is associated with higher occurrence and recurrence rates of neural tube defects. The American Academy of Neurology (AAN) and American Epilepsy Society (AES) recommend that all women with epilepsy of childbearing age receive at least 0.4 mg of folic acid per day. There is currently insufficient evidence to know if higher doses of folic acid offer greater benefit.
EFFECTS OF ANTIEPILEPTIC DRUGS ON COGNITION
In addition to the teratogenicity of AEDs, AEDs may also affect cognitive outcomes of exposed infants. Retrospective and prospective studies suggest a negative effect of valproate; children whose mothers took valproate have lower scores on neuropsychological tests and have more special needs. Early studies were limited by retrospective design, size, and inability to control for confounding variables such as maternal IQ. Data from the prospective wellcontrolled Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study finds that in utero valproate exposure was associated with impaired cognitive development. Children exposed to valproate had significantly lower IQ scores at age 6 years (analyses controlled for maternal IQ, AED dose, gestational age, and folic acid use) when compared to children whose mothers took carbamazepine, lamotrigine, or phenytoin. In addition, findings from NEAD suggest that verbal abilities may specifically be at risk from valproate as well as the other studied AEDs (carbamazepine, lamotrigine, and phenytoin). Periconceptional folic acid use resulted in improved cognitive outcomes. The mean IQs were higher in children exposed to periconceptional folic acid (mean 108, 95% CI 106 to 111) when compared to unexposed children for all AEDs (mean 101, 95% CI 98 to 104; P =.0009). Valproate exposure has also been reported to increase the risk of autism and autism disorders among in utero exposed children. Cognitive outcome data is limited for other commonly used AEDs.
MANAGEMENT OF EPILEPSY DURING PREGNANCY
Management of epilepsy in women of reproductive age should focus on maintaining effective control of seizures while minimizing fetal exposure to AEDs. This applies to dosage and number of AEDs. Medication reduction or substitution should be considered prior to conception. Altering medication during pregnancy increases the risk of breakthrough seizures and exposes the fetus to an additional AED. The recommended AED management in pregnancy is monotherapy at the lowest effective dose. Higher AED doses are associated with higher risks of MCMs as well as poorer cognitive outcomes. If there is a family history of neural tube defects, an agent other than valproate and carbamazepine should be considered.
Seizures in pregnancy may result in miscarriage, preterm labor, fetal bradycardia, and injury to the mother and child. Seizures do not appear to increase the risk of MCMs. Frequent generalized tonic-clonic seizures increase risk of impaired cognitive development.
Once a woman is pregnant, prenatal diagnostic testing includes a maternal serum α-fetoprotein (AFP) and an anatomic ultrasound at 14 to 18 weeks. This combination will identify more than 95% of infants with neural tube defects. In some instances, amniocentesis may be indicated.
Enzyme-inducing AEDs may result in AED-related vitamin K deficiency, which increase the risk of early fetal hemorrhage. Although earlier AAN guidelines recommended that women taking enzyme-inducing AEDs be given supplemental vitamin K (vitamin K1, 10 mg/day) for the last month of gestation, a recent review concluded that there was insufficient evidence to determine if prenatal vitamin K supplementation reduces neonatal hemorrhagic complications. Importantly, all neonates receive vitamin K at delivery.
For pregnant women with new-onset seizures, the diagnostic strategy is similar to that for any patient with a first-time seizure. The neurologic history and examination can be directed to signs of a specific cause, such as acute intracranial hemorrhage or central nervous system (CNS) infection. Evaluation should include screening for hypertension, proteinuria, and edema to exclude eclampsia. Follow-up studies include serologic tests for syphilis and HIV, electroencephalogram (EEG), and magnetic resonance imaging (MRI), which is the preferred imaging technique for pregnant women. As in nonpregnant women with a first-time seizure, treatment depends on seizure type and cause.
PREECLAMPSIA AND ECLAMPSIA
Preeclampsia and eclampsia are most often seen in young primigravida women and in multiparous women with a change in partner. Preeclampsia is a multisystem disorder that is diagnosed clinically by hypertension, proteinuria, and edema. Modern consensus definitions define preeclampsia as pregnancy-induced hypertension beginning after 20th week with proteinuria. Women are also defined as having preeclampsia if they have pregnancyinduced hypertension without proteinuria if they have other common symptoms including cerebral symptoms, epigastric or right upper quadrant pain with nausea or vomiting, or thrombocytopenia and abnormal liver enzymes. Preeclampsia is associated with hepatic and coagulation abnormalities, hypoalbuminemia, increased urate levels, and hemoconcentration. Studies suggest that secreted antiangiogenic factors such as fms-like tyrosine kinase 1 contribute to the acute symptomatology and potential long-term effects. Women who develop preeclampsia/eclampsia should be screened for glomerular and cardiovascular disease throughout life.
EPIDEMIOLOGY
Preeclampsia can progress to eclampsia, a syndrome of diffuse multifocal brain edema that can result in posterior reversible encephalopathy syndrome (PRES; see also Chapter 43). Fewer than 5% of women with preeclampsia progress to eclampsia. The incidence in Europe and other developed countries is 1 per 2,000. In developing countries, the incidence varies from 1 in 100 to 1 in 1,700. Worldwide, eclampsia probably accounts for 50,000 deaths annually. The main cause of death is pulmonary edema.
CLINICAL FEATURES
Neurologic abnormalities associated with eclampsia may include headaches, confusion or lethargy, seizures, cortical blindness or visual field defects, coma, brain hemorrhage, or death due to herniation from massive global cerebral edema. Seizures are most often generalized but may be partial. Common neurologic findings on exam include memory deficits, increased deep tendon reflexes, visual perception deficits, visual information-processing deficits, altered mental status, and cranial nerve deficits. Cortical blindness and visual field defects may occur because occipital lobes are preferentially involved.
DIAGNOSIS
The differential diagnosis of eclampsia includes subarachnoid hemorrhage and cerebral venous thrombosis. The diagnosis is established by appropriate clinical features in the presence of increased blood pressure plus proteinuria, edema, or both. A significant increase in blood pressure is defined as an increase of more than 15 mm Hg diastolic or 30 mm Hg systolic above baseline measurements obtained before or early in pregnancy. If no early reading is available, a blood pressure of 140/90 mm Hg or higher in late pregnancy is significant.
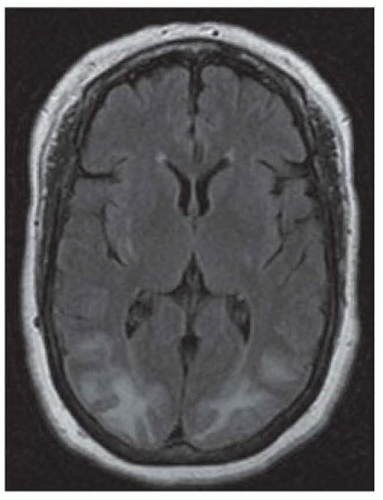
Neuroimaging, EEG, cerebrospinal fluid (CSF) analysis, and angiography may help in diagnosis. Computed tomography (CT) is usually normal in eclampsia but may show hypodense regions in areas of cerebral edema. MRI provides better detection of edema in the cortical mantle and is the mainstay for confirming the diagnosis. MRI characteristically shows sulcal hyperintensity and small multifocal microinfarcts and hemorrhages, with a predilection for the occipital lobes (Fig. 124.1). EEG may show spike-and-wave discharges. The CSF is usually normal in preeclampsia but the protein content may be moderately elevated, and the pressure may be increased. In some patients, angiography shows arterial spasm. A subset of patients with severe eclampsia also develop HELLP syndrome.
Pathologic examination of eclamptic brains reveals petechial hemorrhages in cortical and subcortical patches. Microscopically, these petechial hemorrhages are ring hemorrhages about capillaries and precapillaries occluded by fibrinoid material. Areas that are predisposed include the parietooccipital and occipital regions.
TREATMENT
The most accepted treatment of eclampsia is delivery of the fetus, if possible. Hypertension should be treated with continuous infusion antihypertensive agents, initially directed toward attaining the patient’s baseline premorbid blood pressure levels or a 20% reduction in systolic blood pressure, whichever is higher. Presently, magnesium sulfate (a 4- to 6-g loading dose, followed by an infusion of 1 to 2 g an hour, with a target serum magnesium level of 2.0 to 3.5 mmol/L) is the first-line treatment for the prevention and treatment of eclamptic seizures and other symptoms of eclampsia. Randomized trials have compared magnesium sulfate and other agents including phenytoin and diazepam, and the results suggest that magnesium sulfate is the agent of choice. An international randomized placebo-controlled trial (MAGPIE trial) compared the use of magnesium sulfate and placebo for preeclampsia; magnesium sulfate halved the risk of eclampsia and reduced the risk of maternal death from 1.9% to 0.8% [Level 1].1 Hypertension refractory to magnesium alone can be treated with intravenous nicardipine, clevidipine, or labetalol. There were no substantive harmful effects to mother or baby in the short term. For women at high risk for preeclampsia, the U.S. Preventive Services Task Force recommends lowdose aspirin as preventive medication after 12 weeks of gestation [Level 1].2
STROKE
EPIDEMIOLOGY
Pregnancy is a risk factor for stroke, and the postpartum period is the most vulnerable time. The reported incidence of stroke secondary to pregnancy varies between 4 and 34 deliveries per 100,000. Data from the National Inpatient Sample of the Healthcare Cost and Utilization Project suggest that the incidence of pregnancyassociated stroke has risen since the 1990s. The analysis, which included all types of pregnancy-associated strokes found a 47% when comparing the time periods 1994-1995 to 2006-2007. Concurrent hypertensive disorders and heart disease explain most of the increase. Mortality is approximately 10% to 13% and is higher in black women, older women, and those with no prenatal care. Factors that increase the risk for stroke include pregnancyrelated hypertension and cesarean delivery. Certain medical conditions may also increase the risk (Table 124.2). Presumptive mechanisms include changes in the coagulation and fibrinolytic systems leading to a hypercoagulable state and an increase in viscosity and stasis, which can promote thrombosis. In the postpartum period, the large decrease in blood volume at childbirth, rapid changes in hormone status that alter hemodynamics and coagulation, and the strain of delivery may predispose to a stroke. The risk of thrombosis in the postpartum period persists until at least 12 weeks after delivery.
ISCHEMIC STROKE
Arterial occlusion resulting in cerebral infarction (see also Chapter 35) approximately one-half strokes in pregnant women. Arterial occlusion occurs primarily in the second and third trimesters. Arterial strokes are generally a consequence of identifiable risk factors, including premature atherosclerosis, moyamoya disease, Takayasu arteritis, fibromuscular dysplasia, and primary CNS vasculitis.
CEREBRAL VENOUS THROMBOSIS
Cerebral venous thrombosis (see also Chapter 40) is the second most common cause of stroke in pregnant women, causing approximately one-third of strokes in pregnant women. Cerebral venous thrombosis usually occurs late in pregnancy or in the postpartum period. The pathophysiology of venous infarctions differs from strokes associated with arterial occlusion, as they occur in the setting of venous thrombosis secondary to venous congestion and rise in pressure. Contributing factors include the underlying hypercoagulable state in pregnancy, alterations in platelet function, prothrombotic and antithrombotic proteins, as well as iron deficiency anemia and adaptive response to hemorrhage of labor and delivery. Testing for free protein S deficiency, an acquired hypercoagulable state, is positive in many patients. The clinical presentation is variable, as the woman can present with headaches (the most common presenting symptom), focal neurologic deficits, depressed mental status, or seizures. Hematologic disorders can play an etiologic role in arterial and venous strokes (see Table 124.2). Other causes are cardiogenic and paradoxic emboli.
TABLE 124.2 Medical Conditions that Increase the Risk of Stroke in Pregnancy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
DIAGNOSIS
The key to early diagnosis of stroke in pregnancy is prompt neuroimaging. As with nonpregnant patients, CT and MRI should be done to identify areas of stroke as well as study cerebral vasculature. Angiography including magnetic resonance (MR), CT, or transfemoral catheter angiography may be needed to assess stroke etiology. The current gold standard for diagnosing cerebral venous thrombosis is magnetic resonance venography (MRV) in combination with MRI. Imaging of cerebral venous thrombosis reveals thrombus within cerebral vein or venous sinus either without parenchymal changes or with evidence of cerebral edema, apparent ischemic stroke, or hemorrhage. Hemorrhage occurs in almost half of those with cerebral venous thrombosis (see hemorrhage section for further discussion). Further evaluation such as cardiac ultrasound and serologic studies to evaluate for conditions associated with an increased stroke risk should be pursued. With the exception of protein C, protein S, and antithrombin III deficiency, other hypercoagulable studies can be performed. Evaluation of protein C, protein S, and antithrombin III factors should occur at least 6 weeks after delivery, as they are directly affected by pregnancy itself.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree