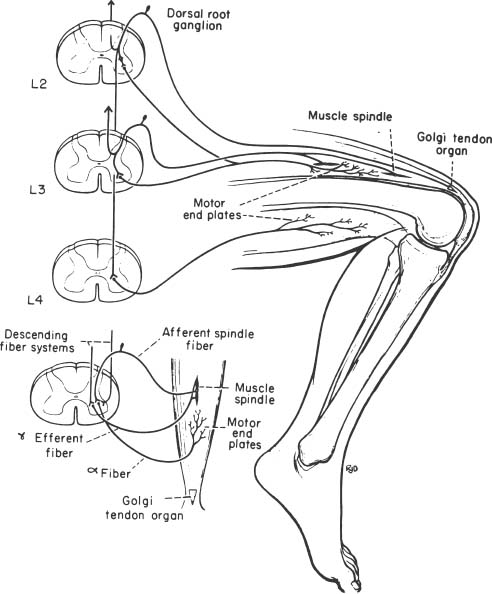
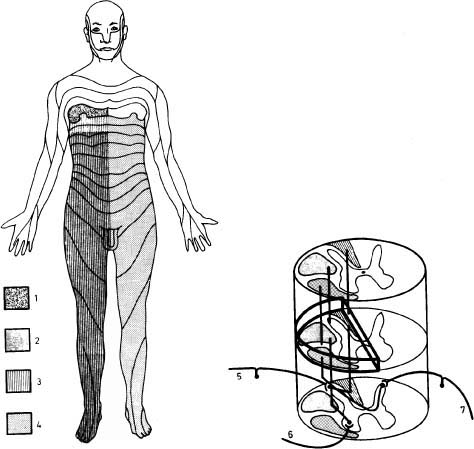
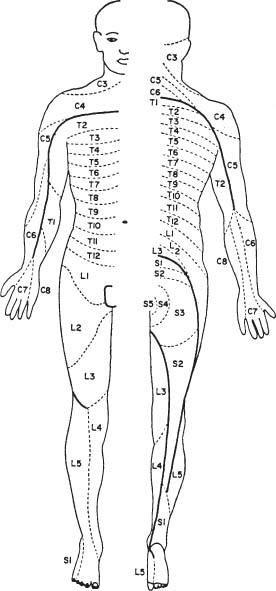
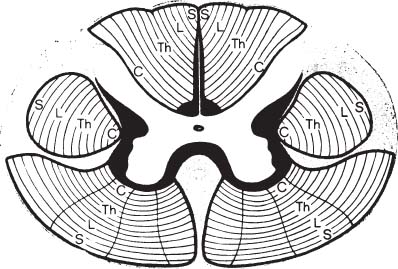
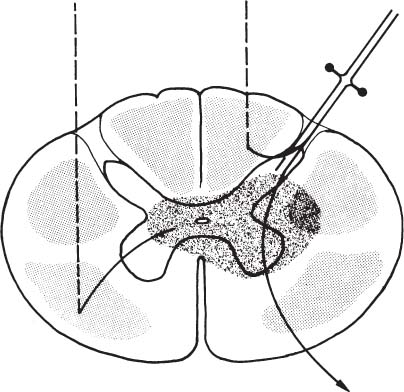
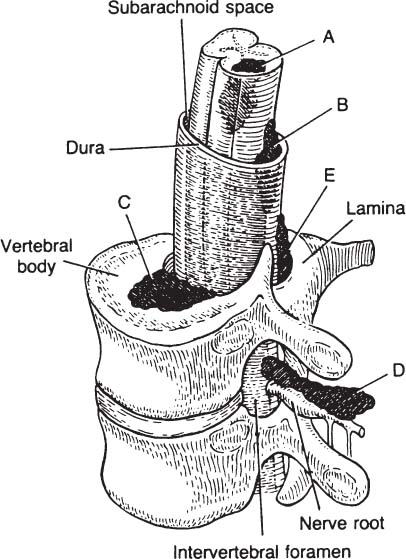
6 The diagnosis of tumors of the spinal canal and peripheral nerves requires a masterful knowledge of the anatomy and physiology of the spinal cord and peripheral nervous system and familiarity with the typical clinical manifestations of the diseases that can involve these neural structures. The segmental organization of the spinal cord and its roots permits astute clinicians to accurately localize the level and extent of spinal cord dysfunction produced by space-occupying lesions of the spinal canal. Distinguishing spinal cord from peripheral nerve dysfunction can be a considerable challenge to clinicians’ skills. Knowledge of the signs and symptoms of long tract involvement, the dermatomal and myotomal organization of the spinal cord, and the sensory distribution and motor innervation of peripheral nerves enables clinicians to identify and localize neurologic dysfunction to the spinal cord or nerve. Moreover, the evolution of the disease process can assist clinicians in distinguishing malignant tumors of the spinal canal from benign lesions. For instance, benign growths such as spinal meningiomas typically produce gradual spinal cord dysfunction and subtle clinical signs over the course of years. In contrast, a rapidly growing malignant lymphoma of similar dimensions can produce paraplegia over weeks or months. An early diagnosis is of particular importance for tumors of the spinal canal because most of these lesions arise from the vertebrae, spinal membranes, or roots and thus can be approached surgically before spinal cord compression and its usually irreversible clinical manifestations develop. This chapter reviews the symptoms and signs of spinal cord and nerve dysfunction produced by tumors involving these structures and presents the clinical manifestations of spinal cord and nerve diseases so that clinicians can localize disease to the spinal cord at various levels and differentiate between intramedullary and extramedullary lesions. The spinal cord is divided into 31 segments, each segment represented by one pair of spinal roots. Spinal roots innervate distinct regions of the body, supplying motor, sensory, and autonomic innervation to each region. The sensory area innervated by each root is defined as a dermatome, the skeletal muscles innervated as a myotome, and the blood vessels receiving autonomic innervation as an angiotome.1 The dorsal root is a conduit for sensory impulses to the spinal cord, and compression of this structure causes sensory symptoms, most commonly pain but also sensory loss.1 Damage to dorsal nerve roots can irritate the neurons, causing them to fire spontaneously or inappropriately. These misfires can cause the sensation of pain or paresthesias or otherwise cause innocuous stimuli to be perceived as painful. Pain resulting from dorsal nerve root irritation is usually described as sharp and lancinating and can be associated with localized backache that radiates to the cutaneous region innervated by the affected sensory root. Radicular pain is often aggravated by activities that stretch the involved root or by maneuvers such as coughing or straining. Paresthesias in the area innervated by the nerve root are common, as is tenderness of underlying connective tissues and muscles. Familiarity with the dermatomal map is critical for localizing sensory complaints to dorsal root dysfunction (Fig. 6-1); however, dermatomal maps vary considerably among individuals, and recent neurophysiologic studies have shown that a given dorsal root supplies afferent fibers to as many as five contiguous dermatomes.2,3 Furthermore, autonomic afferents constitute ~10% of dorsal root axons.4 Thus visceral pain occasionally can be referred to the corresponding somatic dermatome. These realities can be a source of diagnostic confusion when sensory deficits alone are used to localize dysfunction to a specific dorsal root or spinal segmental level. Dermatomal variability and overlap appear to be greater for light touch than for pinprick. Consequently, deficits of sensation for pinprick are more reliable when evaluating patients for sensory complaints and for localizing a sensory deficit to a specific nerve root. FIGURE 6-1 Human dermatomal map. (From Sinclair D. In: Jarrett A, ed. The Physiology and Pathophysiology of the Skin, vol. 2. London: Academic Press, 1973:11, with permission.) Dysfunction of the ventral roots of the spinal cord usually manifests as weakness.5 The ventral roots carry motor fibers that innervate muscle groups and can cause diminished muscle tone and bulk when injured. Weakness is the most common consequence of acute ventral radiculopathy; however, because most muscles receive motor innervation from several adjacent roots, weakness may be hard to demonstrate clinically if a monoradiculopathy is present. Chronic radiculopathies result in changes in the excitability of the muscle that can be observed as a subtle rippling of muscle fibers, known as fasciculations. Fasciculations are the result of spontaneous contractions of groups of muscle fibers innervated by one motor neuron. In the event of a chronic monoradiculopathy, fasciculations will occur in a myotomal distribution. Often, electrophysiologic testing reveals subclinical fasciculations of affected muscles. With chronic denervation, muscle atrophy also develops, but it may not be detectable given most muscles are innervated by multiple roots. A sensation of pain often accompanies injury to ventral roots and occurs because ventral roots carry a small number of sensory afferent fibers.6 The character of this pain differs from the pain associated with the irritation of dorsal roots and has been described as a diffuse aching pain of innervated muscles.7,8 Preganglionic autonomic fibers are carried by ventral roots to their synapse in autonomic ganglia. Consequently, autonomic dysfunction can accompany ventral radiculopathy. It is most often encountered when multiple contiguous ventral roots are irritated and can manifest as disturbances of sweating or as a Horner’s syndrome if the C7-T2 ventral roots are affected.9 Pathologic alteration of the deep tendon reflexes can be of enormous value in localizing diseases of the spinal cord. The deep tendon reflex is a monosynaptic reflex involving both the dorsal and ventral roots of a given spinal segment (Fig. 6-2). Dysfunction of either ventral or dorsal root results in a focally hypoactive reflex that suggests specific radicular disturbance. Ascending and descending tracts within the spinal cord modulate the amplitude of these reflexes. Reflexes increase below the level of spinal cord dysfunction. For instance, a lesion at C6–C7 that disrupts the C7 root and compresses the spinal cord at this level would be expected to diminish the triceps reflex and to exaggerate deep tendon reflexes in the legs. The motor and sensory examination is important in distinguishing radicular from peripheral nerve dysfunction. Knowledge of the dermatomal map, sensory distribution of peripheral nerves, and motor innervation of muscles by nerve roots and peripheral nerves is indispensable for localizing disorders to the root or nerve. Dysfunction of a single nerve root rarely results in weakness of a given muscle because most muscles receive motor innervation from several adjacent roots. In contrast peripheral nerve disorders usually cause significant weakness in a group of related muscles. As mentioned earlier, the dermatomal overlap for tactile stimulation is significant. Thus, dysfunction at the level of a single dorsal root may produce minimal and variable loss of tactile sensation. Peripheral nerve injuries, however, usually result in discrete sensory impairment of all modalities, including light touch, in a characteristic cutaneous distribution.1 Finally, autonomic dysfunction, usually manifested as loss of sweating, is associated with peripheral nerve lesions and not disorders of nerve roots because postganglionic autonomic neurons arise from ganglia outside the nerve root where they receive input from several spinal segments.1 They subsequently join peripheral nerves to supply sweat glands, blood vessels, and other structures. It is imperative to diagnose early dysfunction of the spinal cord that manifests with symptoms and signs of long tract impairment because these abnormalities are reversible only if they are diagnosed rapidly. Motor weakness attributed to spinal cord dysfunction can manifest as a lower motor neuron-type weakness if spinal roots are compromised or as upper motor neuron spastic weakness if the corticospinal and associated tracts are impaired. The distribution of weakness, accompanied by appropriate changes in tone and deep tendon reflexes, can help clinicians to distinguish these two types of motor impairment. Classically, weakness due to spinal cord dysfunction is associated with limb hypertonicity, increased reflexes, and a Babinski sign. Typically, spastic paresis of a limb initially manifests as weakness that follows prolonged activity or exercise. Early symptoms include a sensation of heaviness in an extremity such as a leg that interferes with walking. This symptom must be distinguished from intermittent vascular claudication, where patients are likely to complain of cramping pain in the legs with ambulation.1 The pattern of weakness is an important clue to the presence of spinal cord dysfunction. In patients who have quadriparesis or paraparesis, localization of dysfunction to the spinal cord is usually easy. Similarly, patients who develop unilateral weakness involving the face, arm, and leg have disease in the brain. Occasionally, patients with high cervical lesions exhibit unilateral arm weakness before developing findings in the ipsilateral leg. These cases can cause confusion because rarely, a small cerebral lesion produces these symptoms.10–12 However, the presence of neck pain, lower cranial nerve dysfunction, and ultimately contralateral weakness localizes dysfunction to the high cervical spinal cord. Although sensory complaints are often vague and subjective, sensory symptoms attributed to spinal cord dysfunction are common and their description and pattern are often characteristic. Radicular sensory symptoms are often an early complaint associated with compression of segmental spinal roots. Patients with spinal cord lesions involving the posterior columns may complain of paresthesias and numbness involving the soles of the feet or a leg.13 These patients may report a feeling of tightness in an affected extremity. Atypically, impairment of the posterior columns produces a clinical picture in which gait ataxia is the predominant finding.14 Involvement of the spinothalamic tracts produces complaints of extremity or truncal pain described as burning or aching. These symptoms are often vague and can delay diagnosis or lead to misdiagnosis. The ascending posterior columns and spinothalamic tracts are organized somatotopically, with caudal fibers being displaced peripherally within the spinal cord (Fig. 6-3). For this reason, extrinsic tumors that compress the spinal cord will affect sacral and lumbar regions before more rostral segments. This anatomic arrangement can give rise to an ascending sensory myelopathy, where the sacral and lumbar segments are affected before more rostral regions, even by cervical lesions. It can also cause a lesion to be localized too far caudally. A lateral extramedullary lesion can produce a disruption of sensation characteristic of the Brown-Séquard syndrome, where contralateral posterior column deficits are ipsilateral to the lesion and spinothalamic tract dysfunction is contralateral (Fig. 6-4).15 An intramedullary lesion that involves the central cord can cause a suspended and dissociated sensory deficit where pinprick and temperature sensation are diminished or absent, and vibration and position sensation are preserved (Fig. 6-5). FIGURE 6-3 Schematic representation of the lamination of the long tracts of the spinal cord. C, cervical; Th, thoracic; L, lumbar; S, sacral. (From Dennig. Lehrbruch der Inneren Medizin, part II, 8th ed. Stuttgart, Thieme, 1969, with permission). Superficial spinal reflexes include the abdominal reflexes, the cremasteric reflexes, the anal reflex, and Babinski’s sign. These reflexes are caused by superficial stimulation of the skin or mucous membranes. As with deep tendon reflexes, these reflexes can be impaired if the spinal roots that subserve them are affected by disease. Supratentorial centers influence these reflexes via ascending and descending tracts that course through the spinal cord. If these tracts are interrupted, the superficial reflex will be diminished or absent. These reflex abnormalities, particularly if deep tendon reflexes are also exaggerated, point to spinal cord pathology. Superficial abdominal reflexes are elicited by tactile stimulation over the abdomen. The normal response is deviation of the umbilicus to the side of stimulation. T7–T9 nerve roots innervate the upper abdominal reflex above the umbilicus, and T9–T11 nerve roots innervate the lower abdominal reflex. Although spinal cord dysfunction rostral to these segments can abolish these abdominal reflexes, abdominal reflexes can be absent in normal individuals, particularly those who are multiparous or obese or those who have had abdominal surgery. The cremasteric reflex is elicited by simulation of the medial surface of the thigh in males. The normal response is retraction of the ipsilateral testicle, a reflex that is innervated by nerve roots L1–L2. The superficial anal reflex is contraction of the external anal sphincter in response to stimulation of sacral segments S2–S4. Finally, the plantar reflex is critical, and normally one of plantarflexion of the first toe in response to tactile stimulation along the lateral border of the sole of the foot. An aberrant response, Babinski’s sign, is the sine qua non of corticospinal tract dysfunction. In the absence of other signs of an upper motor neuron lesion, Babinski’s sign can occur in a small minority of normal individuals without neurologic complaints.16 Autonomic symptoms and signs that characterize spinal cord dysfunction include impairment of respiration, bowel and bladder dysfunction, and impaired sexual function. FIGURE 6-5 Schematic representation of an intramedullary spinal cord lesion disrupting spinothalamic tracts, sympathetic tract nuclei, sensory afferents, and anterior horn cells. The result is a clinical picture of suspended and dissociated sensory disturbance, with segmental muscle atrophy and depressed reflexes, and loss of thermoregulation and sweating. (From Hornbostel, Kaufmann and Siegenthaler. Innere Medizin in Praxis und Klinik, part II. Stuttgart: Georg Thieme, 1973, with permission.) A functioning diaphragm is crucial for adequate respiration. Spinal segments C3–C5 supply this muscle. A spinal cord lesion above C3 can impair reflex respiration by impairing the descending tracts in the anterior spinal cord. Ondine’s curse, a rare condition in which respiration ceases when a patient falls asleep, occurs when these tracts are impaired, and voluntary control of respiration, which is controlled by fibers that lie in the lateral cervical spinal cord, is preserved.17,18 The anatomic centers that control the bowel and bladder are similar. The bladder is innervated by sympathetic efferents arising in spinal segments L1–L3, parasympathetic efferents from S2–S4, and somatic efferents to the sphincter from S2–S4. Unilateral spinal cord lesions do not disturb sphincter function because descending tracts are bilateral.19 Complete spinal cord transection causes a flaccid bladder and ileus. If the lesion is above S1, the descending pathways that exert control over these autonomic centers and lie in the lateral spinal cord anterior to the corticospinal tract can be affected. A hypertonic reflex bladder results. The dysfunction is characterized by overactivity of the detrusor muscle and sphincter, causing frequent and involuntary emptying of the bladder. Voluntary bowel emptying is also impaired, but anal tone is maintained, as is the anal reflex. Lesions that affect the conus medullaris or cauda equina abolish voluntary control of the bowel and bladder, and a hypotonic bladder with diminished sensation and overflow incontinence results. These lesions also produce fecal incontinence, a flaccid anal sphincter with loss of the anal reflex, and saddle distribution anesthesia. Extramedullary tumors outnumber intramedullary neoplasms by about four to one. They can arise from the epidural space or within the dura (Fig. 6-6).20,21 Extradural tumors are often malignant and metastatic to vertebrae and extend into the spinal canal, where they compress the spinal cord and nerve roots. Occasionally epidural tumors are primary neoplasms (e.g., sarcomas and multiple myelomas).21 Typically, intradural extramedullary tumors are benign growths such as meningiomas and neurinomas; however, large malignant meningeal deposits sometimes cause significant spinal cord and root dysfunction.22–24 Although the definitive distinction between extramedullary and intramedullary neoplasms requires radiographic confirmation, extramedullary and intramedullary tumors have characteristic clinical presentations that assist clinicians in establishing a diagnosis. Radicular pain is a common initial manifestation of extramedullary tumors.11,12,20,25–27 In the case of neurinomas, the pain is usually unilateral and corresponds to specific dermatomes. Meningiomas and other more diffuse intradural tumors are more likely to produce bilateral pain.22,23,28 Lhermitte’s sign is another common clinical finding in patients with extramedullary tumors, but this sign is nonspecific and associated with other afflictions of the spinal cord, including intramedullary tumors, demyelinating disorders, spinal cord trauma, and radiation myelopathy. Weakness also often accompanies the progression of extradural tumors, and its evolution can be characteristic for these neoplasms.29 The rate of progression of weakness, however, does not help to distinguish intramedullary from extramedullary tumors. Weakness usually progresses inexorably, but a remitting and relapsing course has been described with both intramedullary and extramedullary tumors and can be a cause of diagnostic confusion.29 Unilateral extramedullary tumors characteristically produce unilateral spastic weakness. In the case of cervical tumors, the weakness involves the ipsilateral arm and leg to a comparable degree, whereas neoplasms in the thoracic cord produce ipsilateral leg weakness before the contralateral lower limb is affected.30,31 Moreover, ipsilateral radicular weakness of the affected limb may be present at the level of the lesion. Foramen magnum tumors usually produce ipsilateral arm weakness as an early symptom but occasionally cause bilateral arm weakness while sparing the legs.32 FIGURE 6-6 Tumors arising from or metastasizing to the (A) spinal cord, (B) leptomeninges, (C) vertebral body, (D) paravertebral space, and (E) epidural space can compress the spinal cord and cause neurologic dysfunction. (From Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med 1992;327:615, with permission.)
Neurologic Manifestations of Tumors of the Spine, Spinal Cord, and Peripheral Nerves
 Clinical Manifestations of Spinal Segmental Innervation
Clinical Manifestations of Spinal Segmental Innervation
Dorsal Root Symptoms and Signs
Ventral Root Symptoms and Signs
Deep Tendon Reflex Dysfunction
Distinguishing Radicular from Peripheral Nerve Dysfunction
 Symptoms and Signs of Spinal Cord Dysfunction
Symptoms and Signs of Spinal Cord Dysfunction
Motor Symptoms and Signs
Sensory Symptoms and Signs
Spinal Reflex Abnormalities
Autonomic Symptoms and Signs
 Clinical Features of Spinal and Nerve Tumors
Clinical Features of Spinal and Nerve Tumors
Symptoms and Signs of Extramedullary Tumors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree