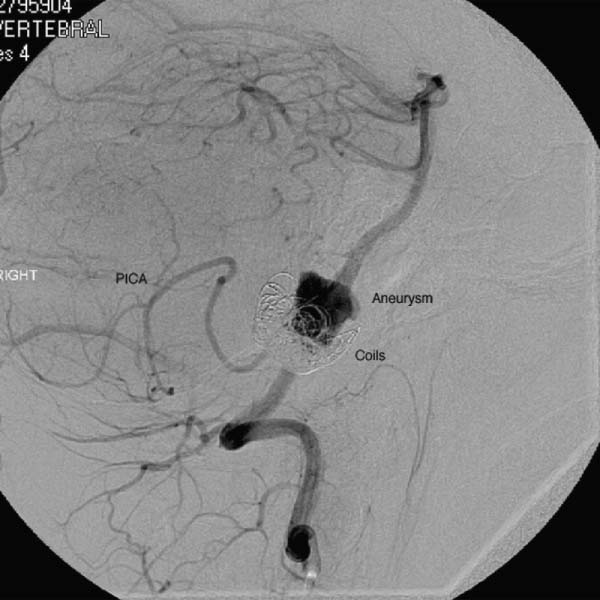
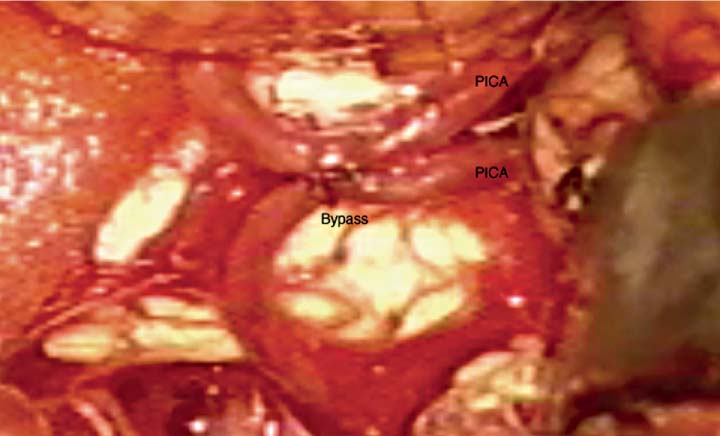
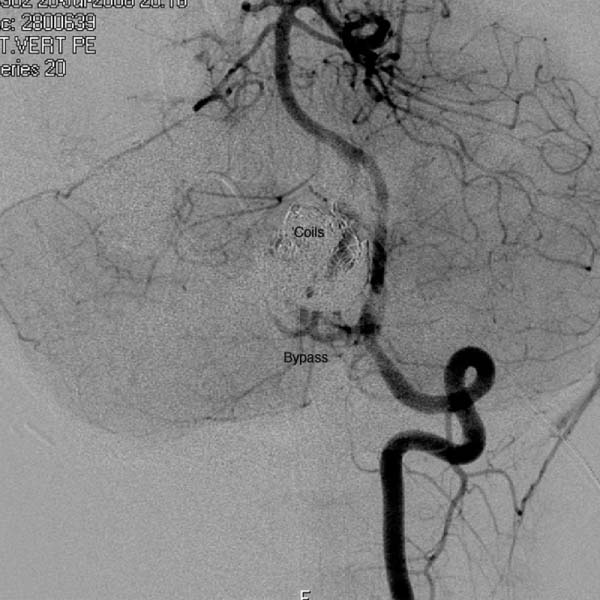
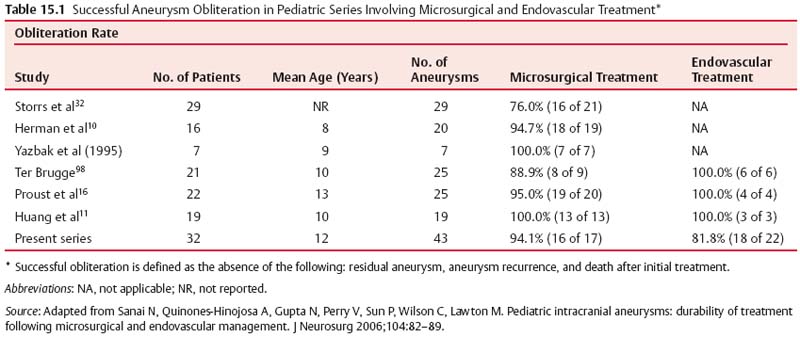
15 Pediatric Aneurysms The first report of aneurysmal subarachnoid hemorrhage (SAH) in a child was published in a German pathology journal in 1871.1 Here, Eppinger detailed a case of a 15-year-old boy who collapsed while exercising. Autopsy revealed an intracerebral hemorrhage associated with an aneurysm and aortic stenosis.2 Several authors have referenced an even earlier report, by Biumi of Milan in 1778, of a ruptured intracranial aneurysm in a child.3,4 This report similarly describes another German youth with aortic coarctation who died suddenly of a ruptured aneurysm while playing football. Interestingly, early thinking associated cerebral aneurysms with a complication of an aortic condition and thus prevented the recognition of the importance of cerebrovascular disease.1 Since then, our understanding of pediatric intracranial aneurysms and cerebrovascular disease has evolved. Aneurysms in the pediatric population are rare, representing ~0.5 to 4.6% of all aneurysms.5,6 Only 700 cases have been reported in the literature,7–17 and these are predominantly case reports. The rarity of pediatric aneurysms was first highlighted by McDonald and Korb in 1939, when they found only 28 pediatric aneurysms out of 1125 (~2.5%) total cases.3Later large population-based reviews supported this early finding. The largest published series of pediatric aneurysms, described by Patel and Richardson,18 included 58 aneurysms in patients younger than 19 years. This summarized the British combined cohort of patients with aneurysms at Queens Square and Atkinson Morley Hospital between 1944 and 1968, when a total of 58 out of 3000 (1.9%) cases of ruptured intracranial aneurysms were recognized in children younger than 19 years. None, however, were younger than 2 years. The rarity of childhood aneurysms is further accentuated when the patients are substratified by age. Rarely have intracranial aneurysms been reported in neonates.19,20 Thus, the exact incidence of pediatric aneurysms is variable, 0.5 to 4.6%, and is more prevalent in the teenage age range and exceptionally rare in younger children.1 In adults, there is a female preponderance; in contrast, boys, particularly during the neonatal and infant periods, are more prone to intracranial aneurysms.14,20–22 Despite the rarity of pediatric intracranial aneurysms, controversy exists regarding their pathophysiologic features. Data on aneurysms in adults suggest that the initial defect is an injury to the internal elastic membrane by hemodynamic forces, which ultimately leads to aneurysm formation.16,23,24 Arterial bifurcations are the most vulnerable to shearing damage because they accept most of the hemodynamic forces, and pathologic analysis confirms that the greatest amount of fenestrations of the internal elastic membrane is at the apex.16 This theory explains the presence of saccular aneurysms in adults and even adolescents.16,22,23 Aneurysms occurring in neonates and children also have vessel wall structural defects; however, these are congenital and related to other disorders. Intracranial aneurysms in children are usually associated with various connective tissue disorders, namely, Ehlers-Danlos syndrome type IV, Marfan syndrome, neurofibromatosis type 1, autosomal dominant polycystic kidney disease, and aortic coarctation, as previously described.25 Lipper and colleagues suggested a large congenital medial defect as an initiating factor for aneurysm development in neonates and children.26 An alteration in parietal connective tissue has been postulated by Ostergaard et al.27,28 Even more, Stehbens and associates observed that an infectious process can also induce fissures in the internal elastic membrane, leading to aneurysm formation.29 Indeed, the incidence of bacterial infections in the pediatric population is estimated to be ~10%, whereas it is only 2.5% in the general population.30 Interestingly, along with blunt head trauma, it has been reported that neonatal birth trauma could potentiate aneurysm formation near the tentorial incisura.31 Although strong evidence exists associating neonatal aneurysms to a congenital defect secondary to other connective tissue disorders, Stehbens et al add to the controversy by defending the assertion that these aneurysms are degenerative hemodynamically determined lesions in children.29 A hemodynamic cause is explained by a force of axial stream on the apex of the vessel at the bifurcation followed by sudden dissipation of kinetic energy, resulting in structural fatigue causing aneurysm formation.16,30 Proust and colleagues observed the preponderance of internal carotid artery (ICA) bifurcation aneurysms in the pediatric population to be 36.4% in their series compared with 2.1% in the adult counterparts. The large ICA bifurcation angle makes it a favorable place for aneurysm formation in children.16 Therefore, we surmise that the early appearance of aneurysms in children reflects a genetic predisposition to the effects of hemodynamic stresses, possibly combined with anatomical susceptibility. In adults, most aneurysms are found in the anterior circulation (anterior communicating artery, posterior communicating artery, and middle cerebral artery [MCA]) and are small; 5 to 15% are noted in the posterior circulation. In contrast, carotid bifurcation is the most common site for pediatric intracranial aneurysms, followed by the posterior circulation. These are often large (1.0–2.5 cm), and 16 to 54% are giant (2.5 cm) aneurysms compared with those found in adults.21 Posterior circulation aneurysms are more prevalent in children than adults by threefold. Lastly, adults are more prone to multiple aneurysms than children.32,33 The initial clinical presentation of a child harboring an intracranial aneurysm is SAH between 50 and 75% of the time, according to most reports in the literature.14,21,34,35 Symptoms of adolescents presenting with SAH are similar to adults with SAH: sudden severe headache, nausea, vomiting, collapse, photophobia, nuchal rigidity, and focal neurologic deficits. Neonates and infants are more likely to present with nonspecific signs, such as irritability, seizures, drowsiness, and vomiting. Generally speaking, the pediatric population with SAH presents in better clinical grades than adults, and the incidence of delayed ischemic deficits secondary to vasospasm is less.10,36 Because large and giant aneurysms are fairly common in children, they often present with signs and symptoms of mass effect, seizures, and obstruction, especially with posterior fossa lesions.21 Because of the rarity of pediatric intracranial aneurysms, their diagnosis can be easily missed. Any child with unexplained neurologic symptoms or unexplained sudden headaches should be investigated thoroughly to rule out intracranial aneurysm rupture. The investigation of a child with a suspected aneurysm or SAH is similar to that for an adult. The work-up is tailored to the patient and could include any combination of computed tomography (CT) scan, magnetic resonance imaging (MRI)/MR angiography (MRA), and lumbar puncture looking for xanthrochromia. Four-vessel cerebral angiography remains the gold standard;21 however, CT angiography (CTA) has proven to be very safe and efficacious in most settings.37 With the recent advancements in diagnostic, microsurgical, and neuroanesthetic techniques, aggressive surgical treatment should always be employed in a pediatric patient with SAH to generate favorable outcome.14,35,36,38–40 In their review of 48 cases, Choux et al noted that 73% of their surgically treated group had an excellent outcome compared with the 7% in the nonsurgical group.41 There is a lower incidence of rebleeding in children (7–13%) when compared with adult controls (20–30%).38,42 Khoo and coworkers espouse early and immediate surgical obliteration of cerebral aneurysm in children with low-grade SAH. Postponement of surgical treatment of high-grade SAH allows for stabilization with respect to edema, hydro-cephalus, and vasospasm, thus lowering surgical morbidity.41 The timing of surgery in patients with high clinical grade SAH must be individualized. The threshold to treat, however, ought to be low in the pediatric patient. The operative management of saccular aneurysms in children is governed by the same principles as in adults, with added concerns of a smaller physiologic reserve in pediatric patients. Extra appreciation of the smaller anatomy and decreased tolerance of temperature changes, blood loss, and large fluid shifts are warranted intraoperatively.36,41 Because of the anatomical variability and typically larger aneurysms in children, surgical obliteration is not straightforward. In a review of the literature, it was observed that direct clip ligation of the aneurysmal neck is possible in only 29.5% of cases.41 Contrary to adults, children often require specialized and innovative techniques to obliterate intracranial aneurysms.10,18,39,43 These include various combinations of clip ligation, entrapment in giant lesions, tandem clipping, angioplastic clipping, aneurysmectomy, microvascular anastomosis and bypass,14 direct excision with reanastomosis, ligation of the parent artery with tourniquet,40 direct ligation of the cervical carotid or vertebral arteries, extracranial-intracranial bypass, and direct excision without bypass.35 Lansen and associates demonstrated that even giant lesions could be treated effectively with micro-surgical techniques. They reviewed 47 cases of giant childhood and adolescent aneurysms; 36 of these lesions were successfully obliterated with surgical occlusion of proximal artery.41 Of these, 29 were completely thrombosed postoperatively. Trapping (six cases), sac resection followed by extracranial-intracranial bypass (three cases), direct neck clipping (two cases), and exploration (two cases) were used in the remainder of patients. Excellent results were obtained in 36 out of 47 cases, with significant morbidity in 6 cases and 5 deaths.41 When treating childhood aneurysms, we must often use creative strategies to individualize treatment to gain a favorable outcome. We describe a recent case at our institution where we effectively treated vertebral artery (VA)–posteroinferior cerebellar artery (PICA) aneurysm with an in situ side-to-side PICA–PICA bypass. An 11-year-old boy with a partially coiled enlarging VA–PICA aneurysm presented with brainstem compression. Figure 15.1 shows the giant aneurysm. Rather than just employing hunterian ligation of the VA and risking a PICA territory infarct in an intact child, we decided to preserve the PICA by revascularization through an in situ side-to-side PICA–PICA bypass due to the lack of an adequate occipital artery donor. Figure 15.2 shows the bypass intraoperatively, and Fig. 15.3 demonstrates a postoperative angiogram showing filling of the medullary segment of the PICA territory and obliteration of the aneurysm. The patient remained intact 15 months later.44 Despite our overall limited surgical experience with childhood aneurysms, various authors have cited certain surgical observations from their experience. Children evidently tolerate proximal arterial occlusion and hunterian ligation far better than adults.40 Successful carotid and vertebral artery ligation with a Drake tourniquet is well known.45,46 Cervical carotid occlusion may be especially effective for control of some carotid-ophthalmic aneurysms or for otherwise untreatable cavernous or petrous aneurysms. Surprisingly, it has also been shown that basilar artery occlusion can be tolerated with few deficits.45 It is unclear whether this is due to a larger collateral supply or increased plasticity of the child’s brain. The rationale for ligation is to reduce the pressure head and filling within the lesion, thereby allowing the sac to thrombose.46 Aneurysmorrhaphy or evacuation of intraluminal clot in such partially thrombosed aneurysms improves preoperative neural compression syndromes.39 Every attempt should be made to avoid vessel deconstruction in young patients due to the concern regarding long-term risks of large vessel sacrifice, although recent literature describes sacrifice to be relatively safe.47 Fig. 15.1 Lateral angiogram of the right vertebral artery revealing a compacted large aneurysm at the vertebral artery–posteroinferior cerebellar artery (PICA) junction. Endovascular treatment of pediatric intracranial aneurysms cannot be ignored. It is an attractive option for obvious reasons. Compared with microsurgical experience for these lesions, endovascular therapy is still in its nascent stage. Fig. 15.2 Intraoperative picture of bilateral caudal loops of the PICA in a side-to-side bypass using 9–0 Prolene suture. The long-term outcomes following endovascular treatment are not fully elucidated in the literature. Longer life expectancies and differences in underlying disease in children with aneurysms raise important issues regarding the durability of treatment choice between microsurgical and endovascular therapy. Direct comparisons between endovascular and microsurgical treatment are starting to emerge in the literature. Sanai and colleagues at the University of California, San Francisco reviewed this question (Table 15.1).48 They retrospectively reviewed a total of 43 pediatric aneurysms treated between 1977 and 2003 at their institution with a focus on treatment durability. Their results show that micro-surgical treatment offered a 94.1% aneurysm obliteration rate compared with 81.8% for the endovascular group (Table 15.2). The authors concluded that both endovascular and microsurgical treatments are generally successful. Microsurgical therapy, however, is more efficacious in completely eliminating the aneurysm and more durable over the extended lifetime of these patients. Furthermore, the coiling of large aneurysms often results in significant recanalization rates, arguing against their use in pediatric patients.48 Fig. 15.3 Anteroposterior angiogram of the left vertebral artery revealing filling of the bypass and the distal medullary segment of the PICA. There is no filling of the aneurysm or the vertebral artery. The overall outcome of pediatric aneurysmal disease is good. Krishna et al reported an 82% favorable outcome in surgically treated cases.49 Children presenting with low clinical grades had 100% favorable outcome. A similar experience has been documented in the literature.9,14–16 Norris and Wallace argued that children usually present in better clinical grades after SAH compared with adults and that overall surgical outcome is better.15 According to Ferrante et al, younger patients tolerate surgery better due to greater brain functional capacity and a robust collateral circulation.16,50 Delayed surgery for higher grades has improved surgical outcome; however, this is at the expense of management morbidity. The incidence of pediatric aneurysms is rare. Thus, our collective experience in managing these lesions is relatively limited and localized at specialized tertiary institutions. However, the current sophistication of microsurgical technique, various revascularization options, neurophysiologic monitoring, excellent imaging modalities, high-level pediatric intensive care, and neuroanesthetic techniques have made the surgical management of SAH in children very safe. The unique pathophysiologic features, along with size and distribution variability of pediatric aneurysms, require creative and innovative surgical strategies to obliterate these lesions. It is imperative to individualize treatment. Indeed, endovascular techniques have become quite sophisticated, and this treatment modality is very effective in selected cases. Controversy regarding the durability of microsurgical versus endovascular repair exists in the literature, and as we become more advanced, there will be more emerging issues that will continue to fuel the debate. As far as we are concerned, the best and safest way to tackle pediatric aneurysmal disease is a combination of microsurgical and endovascular therapy in highly specialized neurovascular centers.
Surgical Management
Incidence
Pathophysiology
Presentation
Surgical Treatment
Outcome
Conclusion
Treatment Group (%) |
|
|
Variable | Microsurgical | Endovascular |
No. of patients | 13 | 16 |
No. of aneurysms | 17 | 23 |
Complete obliteration | 16(94.1) | 18(81.8) |
Recurrence | 0(0) | 3(13.6) |
De novo formation | 1(5.9) | 3(18.8) |
New neurologic deficits | 1(7.7) | 1(6.3) |
Mortality rate | 0 | 0 |
Source: Adapted from Sanai N, Quinones-Hinojosa A, Gupta N, Perry V, Sun P, Wilson C, Lawton M. Pediatric intracranial aneurysms: durability of treatment following microsurgical and endovascular management. J Neurosurg 2006;104:82–89.
Endovascular Treatment
Pediatric intracranial aneurysms account for 5% of all intracranial aneurysms.51–55 Morphologically, they differ from aneurysms seen in adults in that they tend to be larger and more dysplastic, have a wide neck, and more often occur in atypical locations. In addition, intracranial aneurysms seen in children are more often associated with etiologic factors such as trauma, congenital disorders, and infection than in the adult population.52 Intracranial aneurysms may be classified as saccular, fusiform, infectious (“mycotic”), or dissecting aneurysms, each category having its specific natural history, management options, and clinical outcome, as aneurysm morphology is a key factor in regard to the degree of aneurysm obliteration and the rate of recurrence after either microsurgical or endovascular treatment.56
Therapeutic options for pediatric intracranial aneurysms include surgical and endovascular techniques, or a combination of these, with a slow but gradual shift noted over the last 15 years in favor of endovascular treatment.51 This tendency reflects the progresses made in neurointerventional techniques, as well as the promising results observed in the endovascular treatment of adult aneurysms.51 The International Subarachnoid Aneurysm Trial (ISAT), a randomized controlled trial comparing surgical clipping with endovascular coiling for the treatment of ruptured cerebral aneurysms, has shown better outcomes in the endovascular treatment group.57,58 It was found in particular that, for ruptured aneurysms felt to be equally treatable by either method, the relative risk of death or significant disability at 1 year for patients treated by coiling was lower than in the surgical group (absolute risk reduction of 7.4%), whereas the risk of late rebleeding was higher in the endovascular group. The study has been criticized in regard to possible selection biases detrimental to the surgical group, as well as to the level of expertise of the neurosurgeons performing the surgical treatments (general rather than vascular neurosurgeons).59 The ISAT study nonetheless helps support the notion that endovascular therapy is a valid alternative to surgical clipping; although no child was included, it may suggest that embolization could be a safe and effective treatment option for pediatric patients as well. However, endovascular therapy for pediatric intracranial aneurysms, either ruptured or unruptured, requires further evaluation before its exact role can be determined.
Technical Considerations
Endovascular techniques for the treatment of intracranial aneurysms with conservation of the parent artery, also known as constructive therapies, include standard coil embolization, coil embolization with balloon remodeling or stent assistance, and balloon-assisted liquid polymer embolization (Fig. 15.4A–C
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree