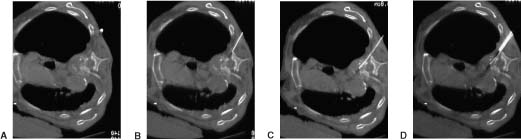
16 Percutaneous diagnostic biopsy procedures of the spine have become increasingly popular over the past decades1–8 for several reasons. These types of procedures tend to be less invasive than more traditional surgical options, generally result in less discomfort for patients, and have been shown to be safe and effective. These are outpatient procedures, which decreases treatment costs. Given the current health care environment and the emphasis on providing high-quality care at minimal cost, it is likely that the popularity of these types of interventions, both for patients and health care providers, will continue to increase. This chapter reviews many of the imaging modalities currently available for the percutaneous biopsy of lesions in the spinal region and peripheral nerves and gives examples of techniques that have proven to be effective. This chapter serves as an introduction to the types of techniques made possible by recent advances in imaging modalities. We wish to emphasize that any type of percutaneous spinal procedure carries significant risk to both the patient and the physician and should be undertaken only by an appropriately trained practitioner. A prudently aggressive approach should be the rule. Current spinal biopsy techniques employ computed tomographic fluoroscopy (CTF), conventional CT, x-ray fluoroscopy, and ultrasound (US) to assist in needle placement during biopsy of the spinal region and peripheral nerves. Each modality has distinct advantages and disadvantages when used for biopsy. There are also specific risks, both to the operator and the patient, associated with each imaging modality. An understanding of the strengths and weakness of each is important prior to utilization during any procedure, especially procedures involving the spine. One of the best ways to avoid complications is to be fully aware of any increased risk that a particular imaging modality may pose relative to another. X-ray fluoroscopy first began to be used widely in the early 1950s. It offers real-time imaging capabilities and relatively short procedure times. It is particularly useful when performing a biopsy of vertebral disc lesions, most vertebral body lesions of the lumbar spine, large paraspinal lesions, and vertebral body lesions of the thoracic spine in which a transpedicular approach can be utilized. However, x-ray fluoroscopy does not provide the spatial resolution of CT and increases the risk of damage to vital structures in the spinal region including major vessels and nerves.9,10 X-ray fluoroscopy thus has limited use when attempting to biopsy small lesions, lesions in the thoracic region in which a transpedicular approach cannot be used, lesions involving the cervical spine; and lesions involving the posterior vertebral arch, epidural space, neural foramen, and peripheral nerves.11 In general, because sonography is unable to penetrate an intact cortex, it has been infrequently used in the biopsy of skeletal lesions, including the spine. And although we have had little experience using sonography as an imaging modality during the biopsy of certain spinal lesions, others have had some success. Gupta et al12 report that sonographically guided fine-needle aspiration biopsy of certain types of lytic lesions has certain advantages. They state that ultrasound is particularly useful in the biopsy of cervical spine lesions in which the lesion has disrupted the cortex of the bone or for lesions that have an extraosseous tissue component. For these types of lesions, sonography is able to penetrate the cortex and provide an image of the lesion, according to the authors. Sclerotic bone lesions and lesions with an intact cortex are not suitable for sonography and would require CT. Sonography offers the possibility of performing biopsies in real time, just like CTF, without the danger of ionizing radiation. Sonography is also portable and typically much cheaper than CTF. Computed tomography first gained popularity as a modality for performing spinal biopsies in the early 1980s and has been shown to be both safe and effective. 13–17 It is commonly used during interventional procedures and offers exceptional contrast and spatial resolution. Furthermore, given the fact that it has been utilized for more than 20 years, many interventionalists are very comfortable overall with the use of this modality; however, unlike CT fluoroscopy, ultrasound, and xray fluoroscopy, conventional CT is unable to provide real-time guidance capability, resulting in longer procedure times as multiple CT scans are needed to confirm appropriate needle or catheter position.18,19 However, conventional CT generally results in less radiation exposure to both operator and patient than either CT fluoroscopy or x-ray fluoroscopy. Conventional CT tends to be most useful in the biopsy of paraspinal soft tissue masses, peripheral nerve lesions, biopsies performed in the thoracic and cervical region in which it is felt that CTF is not needed, and occasionally when small vertebral lesions are being biopsied.20,21 Recent advances in CT technology have led to the development of CT fluoroscopy (CTF). This imaging modalities was first reported by Katada et al22 and Kato et al23,24 in the mid-1990s and has since developed into a powerful imaging tool with widespread application.25–32 CTF combines the benefits of conventional CT with the added value of real-time imaging capabilities and has led to the development of several new treatment options.25–27 Two operational settings or modes can be utilized during CT fluoroscopy: a continuous mode and an intermit intermittent mode. The continuous mode offers real-time imaging capabilities, allowing visualization of the needle tip or catheter throughout the procedure, whereas the intermittent mode provides spot images that allow the operator to periodically locate the needle tip or catheter position in a manner similar to conventional CT.25–27 The two operational modes can be utilized alone or in any combination during interventional procedures, and both have been well documented.25–27 Although the continuous mode offers the optimal visualization during a procedure, it results in greater radiation dosages to both operator and patient.19,20,25–27 As a result, this operational mode is usually reserved for interventional procedures in which a high value is placed on being able to localize the needle or catheter position at all times. For most routine biopsy and drainage interventions, the intermittent mode is used because it provides sufficient spatial resolution and results in less radiation to operator and patient.26–29 A major benefit of CT fluoroscopy is the reduction in needle placement times during interventional procedures when compared with the same procedures performed using conventional CT.26–30 At Johns Hopkins Hospital, procedure times have been reduced by as much as 50%, and reports from other institutions show similar reductions in procedure times when CT fluoroscopy is used.25–29,31,32 The biggest risk associated with CT fluoroscopy is the potential for increased radiation exposure to patients and physicians. Typical radiation exposure factors during CT fluoroscopy are 80 to 120 kVp and 30 to 50 mA per second with radiation dosage rates ranging between 20 and 60 rad/min.20,30 Although Silverman et al27 reported radiation exposure in excess of conventional CT, several other studies have shown that radiation dose to the patient can actually be reduced. Studies conducted at the Mayo Clinic by Carlson et al25 reported a 94% decrease in patient-absorbed dose when CT fluoroscopy was compared with conventional CT. The use of the intermittent technique and low fluoroscopic parameters used during procedures were the primary reasons for this. Several additional studies have also reported the potential for decreased radiation exposure to patients undergoing interventional procedures in which CTF was used.26,33 To help reduce radiation exposure to physicians, several strategies have emerged. The use of lead aprons and lead shields can lower radiation exposure dramatically. Using needle holders during the procedure and thus avoiding placing hands directly into the x-ray beams has also been shown to significantly reduce radiation dosages.30 Currently, at Johns Hopkins Hospital, CTF is being utilized when performing biopsies of the epidural space or neural foramen and of small lesions located anteriorly to the vertebral body, when performing biopsies of the cervical spine, when performing biopsies of the posterior vertebral arch, and when performing procedures in which it is felt to be important to know the needle tip position at all times due to the lesion being located in close proximity to vital structures. In general, complications arise very infrequently, far less than 1%, when spinal biopsies are performed with the proper technique and by a competent interventionalist.34,35 Complications reported include hematoma, vertebral osteomyelitis, radiculopathy, pneumothorax, allergic reactions to drugs, headache, thecal sac disruption, epidural abscess, regional abscess, peripheral nerve damage, paralysis, vascular injury, myelopathy, and disc space disease. We recommend a conservative approach to performing biopsies in the spinal region in general, particularly when such lesions are in close proximity to vital structures, specifically the lungs, major vessels, spinal cord, and nerve roots. The following case examples are provided to illustrate many of the techniques utilized when performing biopsies of the spinal region. For additional guidance in technique when performing spinal biopsies, see Image-Guided Spine Intervention by Fenton and Czervionke.11 Patients with unilateral brachial plexopathy occasionally require biopsy for prognostic and treatment reasons, particularly for patients with a history of breast cancer previously treated with radiation. This case illustrates the biopsy of the brachial plexus lesion using CT guidance.36 Patients with a known neoplasm who present with new unilateral brachial plexopathy first receive a magnetic resonance imaging (MRI) scan, and if abnormal soft tissue is present, CT-guided biopsy is planned. A high-resolution (3 mm or less) contrast-enhanced axial CT scan is obtained during contrast administration from the C5 vertebral level through the inferior axillary level to assist in biopsy planning. Contrast administration is timed to maximize vascular enhancement, as the subclavian vessels are valuable landmarks by which to locate the brachial plexus. The field of view must be large enough to include the anterior and lateral skin surfaces and be centered over the field of interest. The pathologic tissue farthest from the regional neurovascular structure is targeted, and the skin is anesthetized with 2% lidocaine. We avoid deep administration of local anesthetic to allow the patient to comment on the type of pain being felt during the procedure to minimize the chance of damaging brachial plexus neural structures. This also avoids the potential dissection of anesthetic into the perineural space and subsequent entry into the spinal epidural or subarachnoid spaces with subsequent high epidural or spinal anesthesia. A coaxial technique is used, with either a 6-inch, 20-gauge or an 8-inch, 22-gauge calibrated spinal style needle (MSPN 2006 or MSPN 2208, respectively; Manan Medical Products, Northbrook, IL) inserted through a 4-inch, 18-gauge calibrated spinal style needle (MSPN 1804, Manan Medical Products). The shortest anterior or anterolateral approach is taken to the pathologic tissue. Placement of the 18-gauge needle is monitored with CT until it is positioned within 1 to 2 cm of the area of interest, with care taken to avoid vital structures. With the 18-gauge needle in place, multiple tissue passes can be made with the 22-gauge needle with relative safety. The needle is repositioned if paresthesias are experienced by the patient; otherwise, cells can be aspirated for cytologic examination. The use of cutting needles is not advocated, given the increased risk to regional nerves. It is helpful to have a cytopathologist in the CT area who can verify when a diagnostic quantity of tissue has been obtained. Part of each sample is smeared onto slides and stained for immediate microscopic examination by the cytopathologist, and the remainder is rinsed into a solution for cell block preparation (Fig. 16-1). A 62-year-old migrant worker with acute neck pain and fever presented to the emergency room. A mass involving the cervical spine was demonstrated on CT (Fig. 16-2), and CT-guided biopsy of the cervical lesion was recommended to obtain tissue specimens for diagnosis. A high-resolution (3 mm or less) contrast-enhanced axial CT scan should be obtained through the region of interest to assist in biopsy planning. The skin is anesthetized with 2% lidocaine. We avoid deep administration of local anesthetic to allow the patient to comment on the type of pain being felt during the procedure to minimize the risk of damaging neural structures in the region. CT-guided biopsy is then performed using a straight lateral coaxial approach with a simple 16-gauge needle and a 20-gauge Chiba needle, as shown. Tissue samples can then be sent to pathology for analysis and to the lab for culture.
Percutaneous Diagnostic Biopsy Techniques for Tumors of the Spine and Peripheral Nerves
 Imaging Modalities Available for Use When Performing Biopsies
Imaging Modalities Available for Use When Performing Biopsies
X-Ray Fluoroscopy
Ultrasound
Conventional Computed Tomography
Computed Tomographic Fluoroscopy
 Complications of Biopsy of Spinal Region Lesions and Peripheral Nerve Lesions
Complications of Biopsy of Spinal Region Lesions and Peripheral Nerve Lesions
 Case Illustrations
Case Illustrations
Case 1: Biopsy of the Brachial Plexus Region
Technique
Case 2: Cervical Lesion Biopsy
Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




